Abstract
Purpose
Perivascular adipose tissue (PVAT) surrounds the arterial adventitia and plays an important role in vascular homeostasis. PVAT expands in obesity, and inflamed PVAT can locally promote endothelial dysfunction and atherosclerosis. Here, using adipose tissue transplantation, we tested the hypothesis that expansion of PVAT can also remotely exacerbate vascular disease.
Methods
Fifty milligrams of abdominal aortic PVAT was isolated from high-fat diet (HFD)-fed wild-type mice and transplanted onto the abdominal aorta of lean LDL receptor knockout mice. Subcutaneous and visceral adipose tissues were used as controls. After HFD feeding for 10 weeks, body weight, glucose/insulin sensitivity, and lipid levels were measured. Adipocytokine gene expression was assessed in the transplanted adipose tissues, and the thoracic aorta was harvested to quantify atherosclerotic lesions by Oil-Red O staining and to assess vasorelaxation by wire myography.
Results
PVAT transplantation did not influence body weight, fat composition, lipid levels, or glucose/insulin sensitivity. However, as compared with controls, transplantation of PVAT onto the abdominal aorta increased thoracic aortic atherosclerosis. Furthermore, PVAT transplantation onto the abdominal aorta inhibited endothelium-dependent relaxation in the thoracic aorta. MCP-1 and TNF-α expression was elevated, while adiponectin expression was reduced, in the transplanted PVAT tissue, suggesting augmented inflammation as a potential mechanism for the remote vascular effects of transplanted PVAT.
Conclusions
These data suggest that PVAT expansion and inflammation in obesity can remotely induce endothelial dysfunction and augment atherosclerosis. Identifying the underlying mechanisms may lead to novel approaches for risk assessment and treatment of obesity-related vascular disease.
Keywords: Perivascular adipose tissue, Fat transplantation, Endothelial dysfunction, Atherosclerosis, Inflammation
Introduction
Perivascular adipose tissue (PVAT) surrounds most large vessels except the cerebral vasculature and had traditionally been thought to simply provide structural support for blood vessels. Over the past two decades, however, PVAT has become recognized as a physiologically and metabolically active endocrine tissue with important effects on vascular function and disease states [1, 2]. Under physiological conditions, PVAT may exert a protective role against neointimal formation through release of NO and anti-inflammatory adiponectin [3]. However, mounting evidence suggests that in disease states, PVAT contributes to endothelial dysfunction, neointimal formation, atherosclerosis, and other cardiovascular diseases, including abdominal aortic aneurysms, aortic stiffness, and vasculitis [4–12]. Mechanistically, these effects have been linked to local inflammation, oxidative stress, and decreased nitric oxide (NO) release from endothelium along with uncoupling of endothelial NO synthase (eNOS) in PVAT [13, 14].
In rodent models of obesity, PVAT acquires a pro-inflammatory phenotype with macrophage and leukocyte accumulation and production of pro-inflammatory adipocytokines which contribute to migration of vascular smooth muscle cells and myofibroblasts into the neointima [15]. Recent studies suggest that dysfunctional and inflamed PVAT is also associated with metabolic disease, hallmarked by insulin resistance and glucose intolerance. Indeed, in humans, fat surrounding the heart and great vessels expands in obesity, correlating with increased visceral fat mass and insulin resistance [16]. Moreover, under basal conditions, human perivascular adipocytes secrete much higher levels of pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1 compared with other visceral (perirenal) adipocytes [17]. Together, these findings raise the possibility that expansion of PVAT in obesity could also promote vascular disease at distant sites, beyond its well-described function as a local paracrine mediator.
In order to investigate remote effects of PVAT on vascular disease, we adopted an adipose tissue transplantation model. Transplantation of adipose tissues onto arteries is a useful approach to investigate the local impact of PVAT on vascular diseases [5, 18, 19]. Our laboratory previously established a PVAT transplantation model to investigate the role of PVAT in neointimal formation in conjunction with wire injury of the carotid artery [20]. Adapting a similar PVAT transplantation model to the abdominal aorta, this study investigated the systemic and remote effects of PVAT on metabolic disease and atherosclerosis. We found that transplanting just 50 mg of PVAT harvested from obese mice onto the abdominal aorta of recipient hyperlipidemic mice was sufficient to induce endothelial dysfunction and augment atherosclerosis in the remote thoracic aorta. These systemic vascular effects of PVAT were independent of changes in body weight, fat composition, insulin and glucose sensitivity, or plasma lipid levels. Although the precise mechanisms remain to be determined, this study provides the first evidence showing a systemic effect of PVAT on vascular disease.
Methods
Adipose Tissue Transplantation
Using a dissecting microscope, 15 or 50 mg of white PVAT was isolated from abdominal aortas of donor of C57Bl/6J mice fed a HFD (60% kcal fat, D12492, Research Diets, New Brunswick, NJ, USA) for more than 10 weeks. Subcutaneous adipose tissue (SAT), and in some experiments visceral adipose tissue (VAT), was isolated from the HFD-fed mice for use as controls. The isolated adipose tissues were transplanted onto the infrarenal abdominal aortas of 8-week-old male LDL receptor knockout (LDLR−/−, C57Bl/6J background) mice. In brief, after a midline abdominal incision, the abdominal aorta between the left renal vein to the iliac bifurcation was carefully mobilized from the retroperitoneum using microsurgery forceps. Then, after removing the surrounding connecting tissue, adipose tissue was transplanted onto the abdominal aorta and affixed using a synthetic absorbable surgical tissue adhesive (Tissuemend II, Veterinary Products Laboratories, Phoenix, AZ, USA). To account for the impact of surgical manipulation, adipose tissue transplanted mice were compared against sham-operated (non-transplanted) mice otherwise treated identically. After recovering from surgery, the mice were maintained on a HFD for up to 12 weeks, and body weight was monitored weekly. The mice were sacrificed 10–12 weeks after transplantation, and aortas and transplanted adipose tissues were collected for further studies. Endogenous visceral (epididymal) and subcutaneous adipose tissues were carefully dissected and weighed as previously described [21]. Blood was also withdrawn via ventricular puncture and assayed for lipid profiling. Mice were housed at thermoneutrality (30 °C) throughout the study with a 12-h light/dark cycle. All animal studies were registered and approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia at Augusta University.
Nuclear Magnetic Resonance
Fat composition was measured by quantitative nuclear magnetic resonance (NMR) as previously described [21].
Total Cholesterol and Triglyceride Measurement
Total cholesterol and triglyceride were quantified using commercial assays (Wako Pure Chemical Industries, Osaka, Japan) as previously described [22].
Insulin and Glucose Tolerance
Insulin and glucose tolerance tests (ITT, GTT) were performed using glucose strips as previously described [21]. Briefly, at 10 weeks after adipose tissue transplantation, glucose levels were measured in blood obtained from tail veins immediately before and every 10 min after intraperitoneal injection of 0.75 U/kg body weight of human insulin (Humulin R®, Lilly) in mice fasted for 6 h. One week later, glucose levels were also assessed before and every 10 min after intraperitoneal injection of 2 g/kg body weight of glucose in mice fasted for 24 h.
Atherosclerosis Lesion Analysis in Thoracic Aorta
LDLR−/− mice were fed a HFD for 10 weeks after adipose tissue transplantation. After euthanizing mice, the thoracic aorta (from the aortic root to the diaphragm) was isolated and fixed in 4% paraformaldehyde for 24 h, and atherosclerotic lesions were stained with Oil-red O. Images were captured using a microscope digital camera (MU500, AmScope, USA). Quantitative analysis of atherosclerotic lesions was performed using Image-Pro Plus software (Media Cybernetics).
Wire Myography
Thoracic aortas were carefully isolated and cleaned of surrounding tissue, dissected into 2–3 mm of four rings, and mounted on a wire myography as described previously [23]. In brief, two tungsten wires were inserted into the lumen of the arteries and fixed to a force transducer and a micrometer. Arteries were bathed in a physiological salt solution and equilibrated for 60 min. Arterial viability was determined twice with a potassium (KCl)-rich solution (60 mmol/L). Then, dose-dependent constriction curve response to KCl (10 mmol/L to 60 mmol/L) was performed. Endothelium-dependent and -independent relaxation curves were respectively tested with acetylcholine (ACh, 0.1 nmol/L to 100 μmol/L) and sodium nitroprusside (SNP, 0.01 nmol/L to 10 μmol/L). Vessels were preconstricted to 50 to 80% of their maximal 60 mM KCl constriction with serotonin before assessing relaxation responses. Constriction responses to KCl are presented in grams of tension, while relaxation responses to acetylcholine and sodium nitroprusside are expressed as a percentage of constriction induced by serotonin. The relaxation curves were fitted by nonlinear regression analysis, and maximum responses (Emax) were compared amongst the groups.
RNA Extraction and Quantitative RT-PCR
RNA was isolated with a commercially available kit (RNeasy® mini kit, Qiagen), and quantitative analysis of mRNA expression was performed using an MX3000p thermal cycler system and Brilliant II SYBR Green qPCR Master Mix kit (Agilent Technologies). mRNA expression was normalized to the housekeeping gene Arbp and analyzed according to the 2−ΔΔCt method. Primer sequences are listed in the Supplementary Table 1.
Histology
Abdominal aortas with surrounding adipose tissues were fixed in 10% formaldehyde and embedded in paraffin. Paraffin sections were stained with hematoxylin-eosin (H&E).
Statistical Analysis
Data were expressed as mean ± standard error of the mean. Differences between two groups were analyzed by Student’s t test. Multiple group datasets were evaluated for normality, and differences were analyzed by one-way ANOVA followed by Bonferroni post-hoc testing. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 7.0 for Mac OS X (GraphPad Software, La Jolla California, USA).
Results
Adipose Tissues Transplantation in Abdominal Aorta
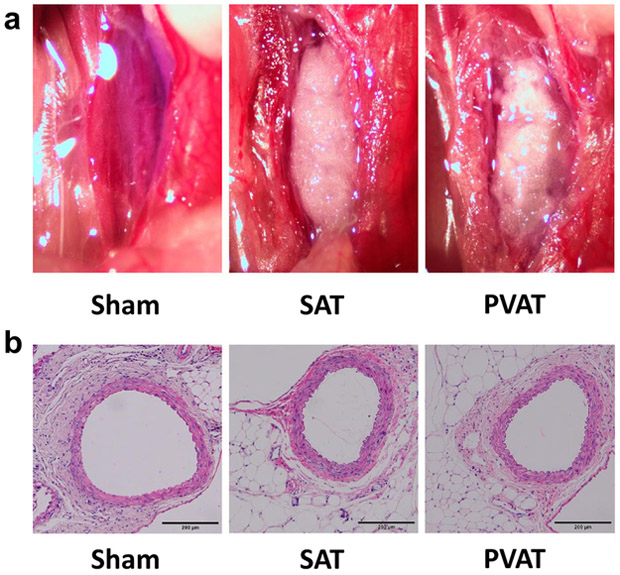
Representative images of donor SAT or PVAT transplanted to abdominal (infrarenal) aorta of recipient LDLR−/− mice are shown in Fig. 1a. Histological analysis confirmed that both SAT and PVAT were well engrafted into the adventitia of recipient aorta at 9 weeks after transplantation (Fig. 1b). The transplanted PVAT exhibited typical histological appearance of white adipose tissue (Fig. 1b).
Fig. 1.
Representative images and H&E staining of donor SAT and PVAT transplanted to infrarenal abdominal aorta. a Adipose tissue transplantation was performed onto infrarenal abdominal aorta, and images taken at the conclusion of the procedure are shown here. b H&E staining of transplanted adipose tissues on abdominal aorta at 10 weeks after transplantation
Adipose Tissue Transplantation Did Not Alter Body Weight, Fat Composition or Lipid Levels in Recipient LDLR−/− Mice
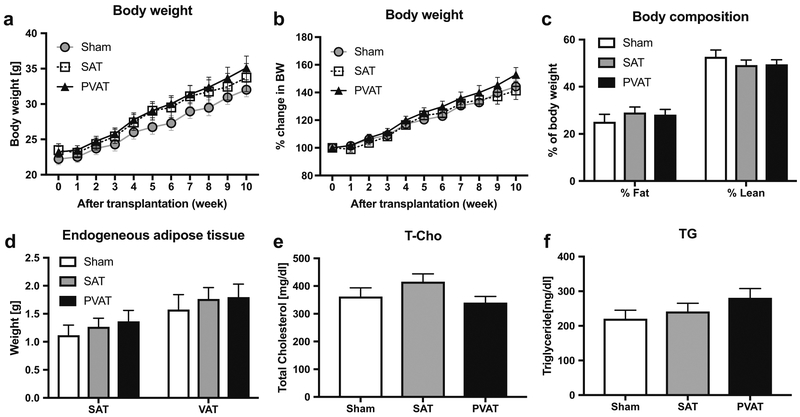
All mice progressively gained weight on the HFD following adipose tissue transplantation, and no significant differences in weight (assessed either by absolute grams or percentage change) were observed amongst the various groups of mice (Fig. 2a, b). Likewise, body composition (fat versus lean mass) measured by nuclear magnetic resonance (Fig. 2c) and endogenous subcutaneous and visceral adipose tissues masses were not significantly influenced by adipose tissue transplantation (Fig. 2d). Lipid analysis at 12 weeks after adipose tissue transplantation demonstrated that levels of total cholesterol and triglycerides in serum were similar amongst the various groups (Fig. 2e, f). These results suggest that neither SAT nor PVAT transplantation affected systemic parameters such as body weight, fat composition, or lipid profile in recipient LDLR−/− mice.
Fig. 2.
Body weight, fat composition, endogenous adipose tissue weights, and serum lipid levels after adipose tissue transplantation. a, b Body weight gain during 60% HFD feeding for 10 weeks after transplantation in the Sham (circle), SAT (square), and PVAT (triangle) groups (n = 14). Absolute weight is shown in (a), and % change in body weight is shown in (b). c Fat and lean mass measured by nuclear magnetic resonance spectroscopy (n = 14). d Endogenous SAT and VAT were harvested and weighed at 12 weeks after transplantation (n = 10). e Plasma total cholesterol (T-Cho) and f triglyceride (TG) levels in mice fed HFD for 10–12 weeks after transplantation (n = 14). c–f Sham (open columns), SAT (gray columns), PVAT (black columns)
Adipose Tissue Transplantation Did Not Affect Insulin Sensitivity or Glucose Tolerance
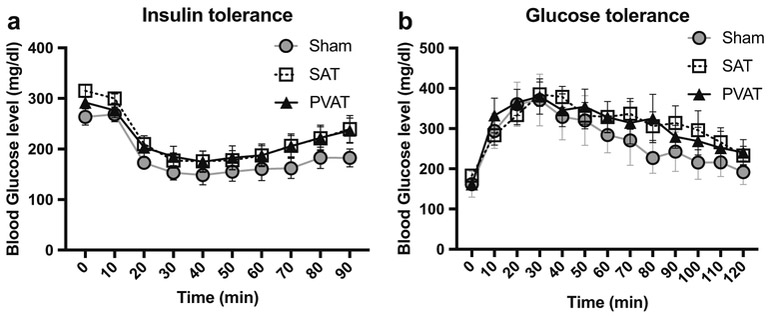
Blood glucose levels prior to intraperitoneal administration of either insulin or glucose were comparable amongst the groups (Fig. 3a, b). ITT demonstrated that the hypoglycemic response to insulin was similar at each time point amongst all groups (Fig. 3a). Hyperglycemic responses to glucose, examined by GTT, were likewise similar amongst all groups (Fig. 3b), suggesting that adipose tissue transplantation did not affect total body insulin sensitivity or glucose tolerance.
Fig. 3.
Effects of adipose tissue transplantation on insulin sensitivity and glucose tolerance. Hypoglycemic responses to insulin at 10 weeks after transplantation (a), and hyperglycemic responses to glucose at 11 weeks after transplantation, in the Sham (circle), SAT (square), and PVAT (triangle) groups (n = 14)
Abdominal Transplantation of PVAT, but Not SAT or VAT, Augmented Atherosclerosis in Thoracic Aorta
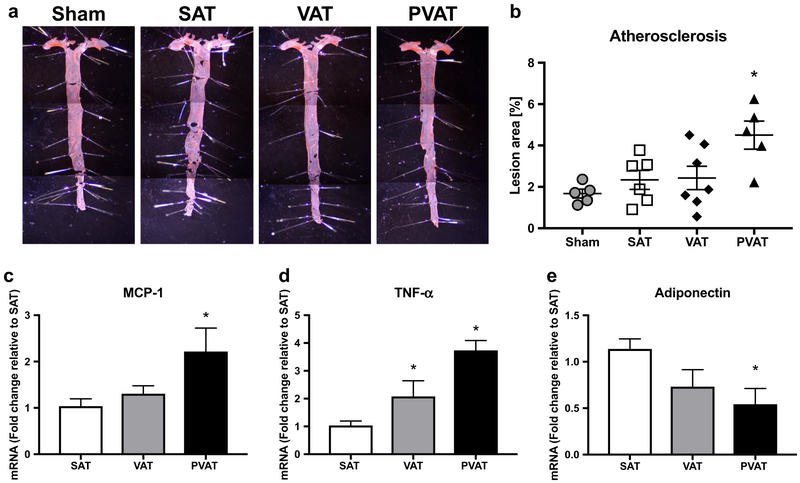
Quantitative analysis indicated that the extent of atherosclerotic lesions in aortic arch and thoracic aorta was significantly higher in the PVAT transplantation group compared with the other groups (Sham; 1.67 ± 0.21, SAT; 2.33 ± 0.45, VAT; 2.43 ± 0.56, PVAT; 4.5 ± 0.68, p = 0.011, Fig. 4a, b). Given that PVAT transplantation did not significantly affect systemic metabolic or lipid parameters, this finding suggested that increased abdominal aortic PVAT might augment thoracic aortic atherosclerosis by promoting inflammation. Interestingly, expression of the inflammatory genes MCP-1 and TNF-α was elevated in transplanted PVAT, while expression of adiponectin, an anti-inflammatory adipokine, was markedly reduced (Fig. 4c–e).
Fig. 4.
Quantification of atherosclerosis in thoracic aorta, and inflammation in the transplanted abdominal aortic adipose tissues. a)After sham procedure or adipose tissue transplantation to the abdominal aortas, thoracic aortas were harvested following 10 weeks of HFD feeding, and atherosclerotic lesions were stained by Oil-red O. Representative whole mount images are shown. b Quantitative analysis of atherosclerotic lesions (*p < 0.05 vs Sham, SAT, and VAT, n = 5–6). c, d, e mRNA expression of adipocytokines in transplanted adipose tissues after 10 weeks of HFD feeding following transplantation (*p < 0.05 vs SAT, n = 5–6)
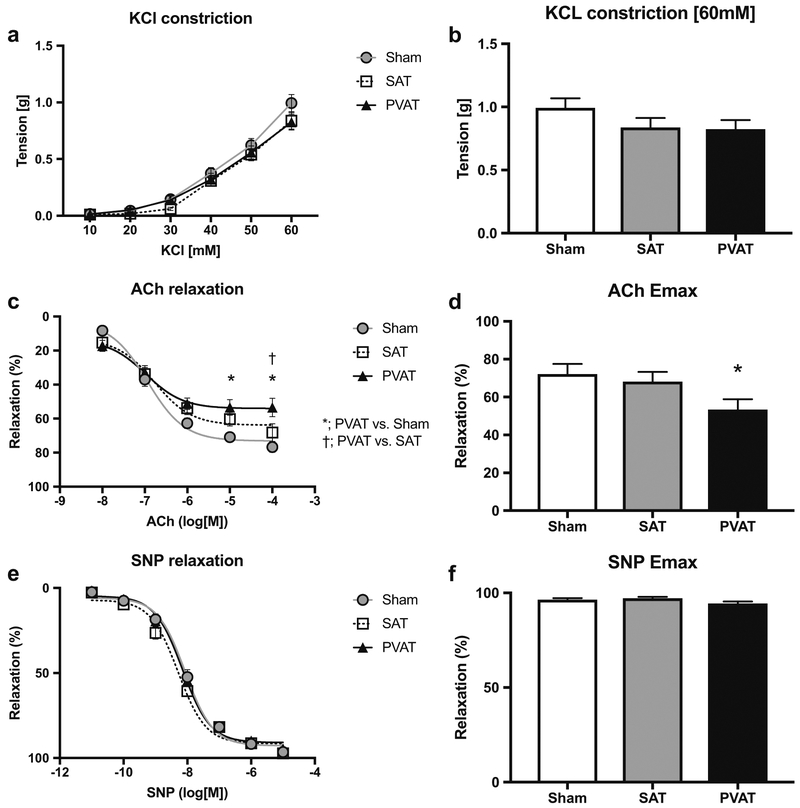
PVAT Transplanted in Abdominal Aorta Promoted Endothelial Dysfunction in Thoracic Aorta
Dose-dependent constriction responses to KCl in thoracic aorta were comparable amongst the groups (Fig.5a, b). Interestingly, following sub-maximal preconstruction by phenylephrine, Ach-induced relaxation (10 μmol/L and 100 μmol/L) was significantly lower in the PVAT transplanted group compared with the sham or SAT transplanted groups (Fig. 5c, d). In contrast, endothelium-independent relaxation induced by sodium nitroprusside was unaffected by PVAT transplantation (Fig. 5e, f), suggesting that increased abdominal aortic PVAT can remotely impair endothelial function.
Fig. 5.
Effects of adipose tissue transplantation to the abdominal aorta on vascular function in the thoracic aorta. a Dose-dependent constriction responses to KCl in the Sham (circle), SAT (square), and PVAT (triangle) groups (n = 9). b Quantitative analysis of vasoconstriction responses to 60 mM KCl. c Endothelium-dependent relaxation responses to acetylcholine (ACh) (n = 9, *p < 0.05 vs SAT, †p < 0.05 vs VAT). d Maximum relaxation (Emax) responses to 100 μmol/L of ACh (*p < 0.05 vs SAT). e Endothelium-independent relaxation responses to sodium nitroprusside (SNP) (n = 9). f)Emax response to 10 μmol/L of SNP
Discussion
PVAT has been recognized as a unique adipose tissue depot, and mounting evidence indicates that both pro- and anti-inflammatory adipocytokines secreted from PVAT regulate vascular homeostasis through paracrine mechanisms. The quantity and pro-inflammatory state of PVAT surrounding atherosclerosis-prone vessels increases in obesity, suggesting that dysfunctional PVAT may contribute locally to atherosclerosis. Here, we demonstrate that transplantation of just 50 mg of PVAT to the abdominal aortas of LDLR−/− mice augments atherosclerosis and promotes endothelial dysfunction at a remote site (the thoracic aorta). These effects were independent of changes in body weight, fat/adipose tissue mass, insulin and glucose sensitivity, and plasma lipid levels. Expression of MCP-1 and TNF-α was significantly increased in the transplanted PVAT, raising the possibility that inflammatory mediators produced by dysfunctional PVAT in obesity could augment systemic vascular disease.
Endothelial dysfunction, characterized by impaired endothelium-dependent vasodilation and a pro-inflammatory state, is a key event in the development of atherosclerosis. Diminished nitric oxide (NO) availability and an imbalance between endothelium-derived relaxing and contracting factors are well established mechanisms leading to endothelial dysfunction. Previous studies have suggested a contributory role of PVAT to endothelial dysfunction. Lee et al. reported that in obesity, PVAT reduces NO production through increased expression of caveolin-1, which negatively regulates endothelial NO synthase (eNOS) [24]. PVAT also may induce endothelial dysfunction via protein kinase C-β dependent phosphorylation and inactivation of eNOS [25]. Inflamed PVAT mediated by a HFD feeding induces eNOS uncoupling both in endothelium and PVAT, which reduces NO production contributing to impaired endothelial dysfunction [13]. However, the aforementioned mechanisms have been linked to local rather than systemic perturbations in endothelial function. Using an adipose tissue transplantation approach, our study demonstrated that PVAT, but not SAT or VAT, can also promote endothelial dysfunction in remote blood vessels. To the best of our knowledge, this is the first evidence demonstrating that dysfunctional PVAT can remotely perturb endothelial function, though the underlying mechanisms remain to be determined.
We have reported that human perivascular adipocytes exhibit a pro-atherogenic phenotype, releasing substantially more pro-inflammatory cytokines as compared with adipocytes derived from other depots (e.g., SAT, omental or perirenal) [17]. MCP-1 is a key regulator of macrophage infiltration into adipose tissue, and MCP-1 expressed in transplanted PVAT promotes neointimal hyperplasia in wire-injured carotid artery [20]. In this study, MCP-1 expression was significantly higher in transplanted PVAT as compared with SAT or VAT, but whether this small amount of transplanted PVAT was sufficient to increase circulating levels of MCP-1, or other inflammatory mediators, is unclear. On the other hand, adiponectin also plays an important role in modulating inflammation, and decreased levels of circulating adiponectin contribute to atherosclerosis and insulin resistance. Previous studies showed that adiponectin released from PVAT plays an important local role in vasorelaxation through paracrine properties [26]. Adiponectin may oppose vascular constriction through phosphorylation of eNOS at Ser1177 to stimulate endothelial NO production [27]. Interestingly, transplantation of PVAT isolated from adiponectin−/− mice onto carotid arteries of apolipoprotein E−/− mice led to accelerated plaque formation [8]. Our data also showed that adiponectin expression in transplanted PVAT was markedly lower than in SAT or VAT at 10 weeks after transplantation, suggesting a potential mechanism of amplification of inflammation in the transplanted PVAT. These results and previous data suggest that an imbalance in pro- and anti-inflammatory mediators in dysfunctional PVAT potentially could remotely promote atherosclerosis as well as endothelial dysfunction, although this hypothesis will require further testing that is beyond the scope of the present study.
In addition, our findings raise a number of important questions. First, the PVAT used for transplantation experiments was harvested from obese mice fed a 60% HFD and maintained under thermoneutral conditions. PVAT in rodents exhibits features of both white and brown adipose tissue depending on the anatomic location, and brown-like function of PVAT has beneficial metabolic effects associated with thermogenesis and fatty acid combustion to ameliorate atherosclerosis [28]. PVAT releases both pro-inflammatory and anti-inflammatory adipocytokines, but when challenged by HFD, this balance is strongly shifted towards inflammation. Indeed, dysfunctional PVAT mediated by HFD plays an important role in atherosclerosis and hypertension [12, 29]. It is entirely possible that transplanting PVAT from mice fed a chow diet, or a HFD with lower fat content, might have produced different (perhaps even protective) effects on endothelial function or atherosclerosis. Likewise, maintaining the mice under ambient temperature conditions might have fostered “browning” of the PVAT to offset the phenotypic changes of the HFD [30]. Such findings would be in keeping with the notion that PVAT has pleiotropic effects on vascular function and may only become pro-atherogenic under certain conditions. Second, the absolute amount of transplanted PVAT required to elicit systemic vascular effects is unknown. In preliminary studies, 15 mg of PVAT was transplanted onto abdominal aorta, and no significant differences were seen in vascular function or atherosclerosis of the aortic arch and thoracic aorta (data not shown), suggesting that a critical amount of PVAT is required. Importantly, researchers should take these findings into account when designing experiments to test the local (paracrine) effects of PVAT on the vasculature. Finally, it remains to be determined whether the systemic effects of the transplanted PVAT were dependent upon a physical association with the vasculature. Additional experiments in which PVAT is transplanted to different locations (vascular vs. non-vascular) will be required to address this question.
In conclusion, this study suggests that HFD-induced inflamed PVAT could contribute to endothelial dysfunction and atherosclerosis in a remote manner, possibly through production of PVAT-derived pro-inflammatory cytokines. These findings support the notion that PVAT is a unique adipose tissue depot that has the potential to profoundly perturb the vasculature under certain conditions, and that targeting PVAT may be a promising therapeutic strategy for treating and preventing obesity-related vascular disease.
Supplementary Material
Acknowledgments
Funding This study was funded by grants HL124097, HL126949, HL134354, and AR070029 from the National Institutes of Health (N.L.W).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10557-018-6821-y) contains supplementary material, which is available to authorized users.
References
- 1.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214(1):3–10. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 2014;3(2): e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai XJ, Li CJ, Chen L, Rong YY, Zhang Y, Zhang M. A hypothesis: adiponectin mediates anti-atherosclerosis via adventitia-AMPK-iNOS pathway. Med Hypotheses 2008;70(5):1044–7. [DOI] [PubMed] [Google Scholar]
- 4.Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM. Effects of obesity on perivascular adipose tissue vasorelaxant function: nitric oxide, inflammation and elevated systemic blood pressure. J Vasc Res 2015;52(5):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res 2009;105(9):906–11. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Komuro I, Sata M. Vascular cells originating from perivascular adipose tissue contribute to vasa vasorum neovascularization in atherosclerosis. Circulation. 2015;132(Suppl_3):A14910. [Google Scholar]
- 7.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2009;29(10):1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Wang Z, Wang C, Ma Q, Zhao Y. Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One. 2015;10(5): e0124031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaue T, Suzuki J, Hamaguchi M, Suehiro C, Tanino A, Nagao T et al. Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension. 2017;70(4):780–789. [DOI] [PubMed] [Google Scholar]
- 10.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 2014;13(3):576–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollan I, Prayson R, Saatvedt K, Almdahl SM, Nossent HC, Mikkelsen K, et al. Inflammatory cell infiltrates in vessels with different susceptibility to atherosclerosis in rheumatic and nonrheumatic patients. Circ J 2008;72(12):1986–92. [DOI] [PubMed] [Google Scholar]
- 12.Öhman M, Luo W, Wang H, Guo C, Abdallah W, Russo H, et al. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol 2016;36(1):78–85. [DOI] [PubMed] [Google Scholar]
- 14.Xia N, Förstermann U, Li H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann N Y Acad Sci 2017;1403:132–41. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Xu T-Y, Guan Y-F, Su D-F, Fan G-R, Miao C-Y. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 2009;81(2):370–80. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 2003;88(11):5163–8. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 2009;104(4):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis: the missing link of adipo-vascular axis. J Biol Chem 2002;277(40):37487–91. [DOI] [PubMed] [Google Scholar]
- 19.Tian Z, Miyata K, Tazume H, Sakaguchi H, Kadomatsu T, Horio E, et al. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J Mol Cell Cardiol 2013;57:1–12. [DOI] [PubMed] [Google Scholar]
- 20.Manka D, Chatterjee TK, Stoll LL, Basford JE, Konaniah ES, Srinivasan R, et al. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arterioscler Thromb Vasc Biol 2014;34(8):1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson TW, Weintraub DS, Crowe M, Yiew NKH, Popoola O, Pillai A, et al. Deletion of the Duffy antigen receptor for chemokines (DARC) promotes insulin resistance and adipose tissue inflammation during high fat feeding. Mol Cell Endocrinol 2018;473:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiang S, Nakatsu Y, Seno Y, Fujishiro M, Sakoda H, Kushiyama A, et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr 2015;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruder-Nascimento T, Kennard S, Antonova G, Mintz JD, Bence KK, Belin de Chantemele EJ. Ptp1b deletion in proopiomelanocortin neurons increases energy expenditure and impairs endothelial function via TNF-alpha dependent mechanisms. Clin Sci (Lond) 2016;130(11):881–93. [DOI] [PubMed] [Google Scholar]
- 24.Lee MH, Chen SJ, Tsao CM, Wu CC. Perivascular adipose tissue inhibits endothelial function of rat aortas via caveolin-1. PLoS One. 2014;9(6):e99947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol 2009;297(1):H460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J 2010;74(7):1479–87. [DOI] [PubMed] [Google Scholar]
- 27.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56(5):1387–94. [DOI] [PubMed] [Google Scholar]
- 28.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 2014;34(8):1621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang L, Milton H, Eitzman DT, Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J 2013;77(1):11–8. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126(9):1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.