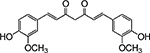
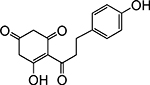
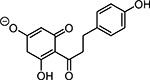
Table 1. Enolates, Enol and Diketo Tautomers of 2-ACP and hytopolyphenols.
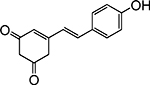
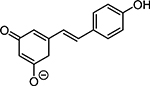
Structures of at least one enol containing tautomer and one diketo tautomer for three plant derived polyphenols and 2-ACP (2-acetylcyclopentanone). In addition, a diketo containing resonance form of the enolate anion from each compound is shown. It should be noted that tautomers exist in temperature and pH dependent equilibrium with each other and their enolates and that the diketo form is generally not the predominant tautomer for 1,3-dicarbonyl compounds. Each of the enolates has additional resonance forms (not shown) that contain anionic oxygen atoms.
| Enol Tautomers | Diketo Tautomers | Enolates | |
|---|---|---|---|
| A. 2-ACP | 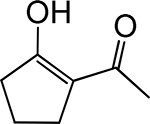 |
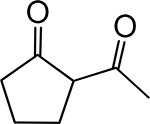 |
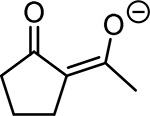 |
| B. Curcumin | 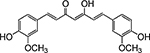 |
 |
 |
| C. Phloretin | 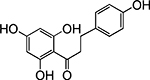 |
 |
 |
| D. Resveratrol | 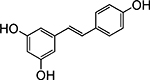 |
 |
 |
