Summary
There are no reliable nonictal biomarkers for epilepsy, electroencephalography (EEG) or otherwise, but efforts to identify biomarkers that would predict the development of epilepsy after a potential epileptogenic insult, diagnose the existence of epilepsy, or assess the effects of antiseizure or antiepileptogenic interventions are relying heavily on electrophysiology. The most promising EEG biomarkers to date are pathologic high‐frequency oscillations (pHFOs), brief EEG events in the range of 100 to 600 Hz, which are believed to reflect summated action potentials from synchronously bursting neurons. Studies of patients with epilepsy, and experimental animal models, have been based primarily on direct brain recording, which makes pHFOs potentially useful for localizing the epileptogenic zone for surgical resection, but application for other diagnostic and therapeutic purposes is limited. Consequently, recent efforts have involved identification of HFOs recorded with scalp electrodes, and with magnetoencephalography, which may reflect the same pathophysiologic mechanisms as pHFOs recorded directly from the brain. The search is also on for other EEG changes that might serve as epilepsy biomarkers, and candidates include arcuate rhythms, which may reflect repetitive pHFOs, reduction in theta rhythm, which correlates with epileptogenesis in several rodent models of epilepsy, and shortened sleep spindles that correlate with ictogenesis.
Keywords: Epilepsy, Biomarkers, pHFOs
Key points.
There currently are no nonictal biomarkers that can be used reliably to diagnose epilepsy, predict its development, or evaluate the effects of antiseizure or antiepileptogenic treatment
Reliable epilepsy biomarkers would greatly facilitate differential diagnosis, revolutionalize approaches to treatment, and streamline new antiseizure and antiepileptogenic drug discovery and validation
To date, the best candidate for an epilepsy biomarker is pHFOs, brief 100–600 Hz EEG events that are recorded directly from the brain, and are believed to reflect synchronously bursting neurons
Basic research on pHFOs is elucidating fundamental neuronal mechanisms of epileptogenesis and ictogenesis
General clinical application of biomarkers for diagnosis and treatment, however, requires noninvasive identification; therefore recent efforts are aimed at recording pHFOs with scalp EEG and magnetoencephalography (MEG), and finding other possible EEG biomarkers
Epilepsy is characterized by the occurrence of epileptic seizures, but not all seizures are epileptic, and not all epileptic seizures are indicative of a chronic epilepsy disease. Diagnosis can be confounded by the presence of psychogenic or physiologic nonepileptic seizures, and two‐thirds of epileptic seizures are natural responses of a normal brain to a transient insult and do not predict subsequent seizure occurrence.1 A tentative diagnosis can be made from electroclinical correlation when ictal events are captured on electroencephalography (EEG), but this expensive, time‐consuming process is not practical to apply to the majority of patients with suspected epilepsy. More useful diagnostic biomarkers should be obtainable when patients are not having seizures.
There are, however, no reliable nonictal biomarkers of epilepsy, in the same sense that hemoglobin A1c is a biomarker of diabetes; therefore there is no test that definitively warrants a diagnosis of epilepsy, or predicts, in people with risk factors such as potential epileptogenic insults or genetic predispositions, who will ultimately develop epilepsy.2, 3, 4, 5 Since its initial clinical application in the mid‐twentieth century, the EEG has been relied upon to demonstrate nonictal epileptiform brain dysfunction with more diagnostic validity in epilepsy than blood tests or neuroimaging. Specifically, interictal EEG spikes can help make a diagnosis of epilepsy, when present, although EEG findings may be normal in many people with epilepsy, and epileptiform spikes can occasionally be seen in people without epilepsy.1 The patterns of interictal EEG spikes can also help to diagnose specific epilepsy syndromes, such as the generalized three‐per‐second spike‐and‐wave of absence epilepsy, and the characteristic centrotemporal spike with a transverse dipole seen in Rolandic epilepsy.1 Recordings of interictal spikes are also used, invasively and noninvasively, to localize the epileptogenic region for surgical resection, and to predict who might be a good surgical candidate6; however, decades of research have failed to identify any other aspects of interictal EEG spikes that correlate with either the severity of epilepsy, or the response to treatment, with the singular exception of the absence epilepsies, where the interictal spike waves are viewed as fragments of three‐per‐second spike‐and‐wave ictal events.1
Need for Biomarkers
Biomarkers that reliably predict ictogenesis—that is, identify the existence of brain areas that are likely to generate epileptic seizures—would obviously be useful for making a diagnosis of epilepsy. This would be of particular importance for patients who present with a single ictal event. Because only 30% of patients presenting with a single ictal event go on to develop epilepsy, in most cases treatment is not instituted until a second seizure occurs, but the next seizure carries a risk of morbidity and even mortality. Consequently, the ability to distinguish between the first seizure of epilepsy and a provoked seizure in a normal brain due to a transient insult that would not be repeated, would have tremendous therapeutic value. Patients could be treated immediately. Similarly, differential diagnosis between psychogenic nonepileptic seizures and epilepsy could be accomplished without the need for expensive and time‐consuming video‐EEG monitoring.
Biomarkers that indicate the severity of epileptogenicity—that is, the degree of likelihood of ictogenesis—could be used to determine the effectiveness of therapeutic interventions, whether pharmacologic or a device such as vagus nerve stimulation (VNS), without the need to wait for another seizure to occur. Elimination of the trial‐and‐error approach to treatment of epilepsy would revolutionize the management of epilepsy diseases. It would be possible, for instance, to rapidly assess the effectiveness of different antiseizure drugs to find the one most likely to render the patient seizure free or to determine that the patient is pharmacoresistant and needs to be considered for alternative treatments, such as surgery. Biomarkers that indicate the severity of epileptogenicity would also greatly facilitate clinical trials of new antiseizure drugs.
There is currently no intervention that prevents the development of epilepsy after a potential epileptogenic insult, such as traumatic brain injury (TBI), intracranial infection, or stroke. The primary reason for this is that it would be prohibitively expensive to carry out a randomized clinical trial to demonstrate the effectiveness of a putative antiepileptogenic intervention. For instance, considering prevention of posttraumatic epilepsy (PTE) following mild to severe TBI, which occurs in only 15 to 20% of patients, would require an extremely large subject population to achieve statistically significant results; and because PTE can take more than 10 years to develop, the trial would need to last years, which would not be economically feasible.2 A reliable biomarker of epileptogenesis, one that would predict with a high reliability those who will develop PTE after TBI, would make it possible to enrich the subject population for a clinical trial, and a biomarker of epileptogenicity that determines who will have epilepsy after the preventive therapeutic intervention, without the need to wait for a seizure to occur, would greatly shorten the trial duration. There is, therefore, a concerted effort to identify biomarkers of epileptogenesis that would make it possible to ultimately identify antiepileptogenic interventions and prevent epilepsy in at‐risk patients. Once preventive approaches become available, biomarkers of epileptogenesis will identify patients who would benefit from this treatment.
Finally, biomarkers of ictogenesis and epileptogenesis could make it possible to develop more cost‐effective rapid throughput screening models for discovery of antiseizure and antiepileptogenic compounds.
pHFOs
In 1999, wideband EEG recordings demonstrated the presence of brief high‐frequency oscillations (HFOs) in the range of 200–600 Hz, which were limited to epileptogenic tissue in patients with mesial temporal lobe epilepsy and animal models of this condition7, 8, 9 (Fig. 1). Since then, attention has been focused on recording pathologic HFOs (pHFOs), not only as indicators of the epileptogenic region for surgical resection, but also as potential biomarkers of epileptogenesis (the development of epilepsy) and of ictogenesis (the diagnosis of epilepsy).
Figure 1.
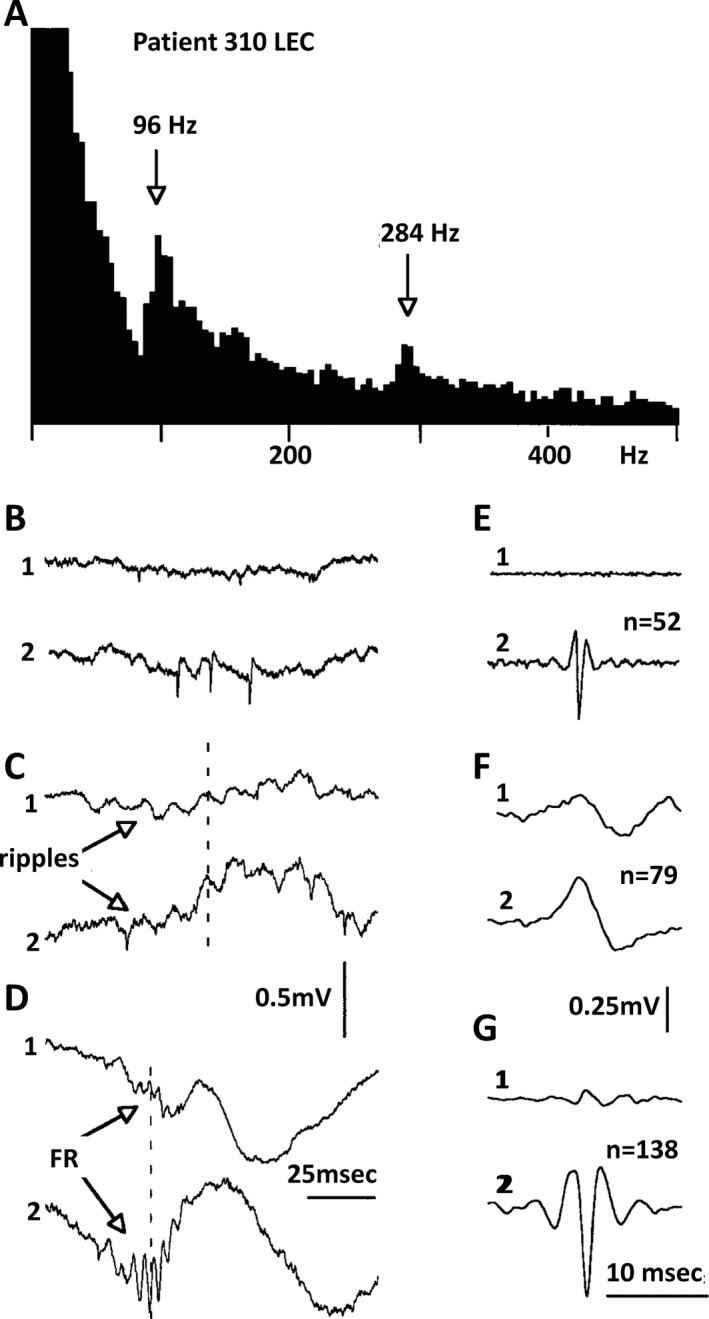
Low and high frequency ripples in human entorhinal cortex. A, Power spectrum of electrical activity recorded from microelectrode 2. Recording bandwidth: 0.1–10,000 Hz.; peaks at 96 and 284 Hz. B–D, Examples of the unit activity, ripples and fast ripples recorded from the same file with 2 electrodes within entorhinal cortex. E–G, Averages of events (the number is indicated in parentheses). Due to similarities of amplitudes, the events were selected into different files by visual estimation. Single unit activity was recorded only from microelectrode 2; note that ripples are in phase on both electrodes and the fast ripples are out of phase. From Bragin et al7, with permission.
The normal hippocampus generates HFOs in the range of 80–200 Hz, termed ripples, reflecting summated inhibitory post‐synaptic potentials (IPSPs), which function to support information transfer by synchronizing activity over broad areas. pHFOs were originally described in the frequency range of 200–600 Hz and were termed fast ripples. Subsequently, it was demonstrated that although normal HFOs reflect summated IPSPs,10 pHFOs represent summated action potentials from synchronously bursting neurons, the hallmark of an epileptogenic region.11, 12 It soon became apparent, however, that frequency alone did not distinguish pathologic from normal events, because the dentate gyrus, which normally does not generate ripples, exhibits ripple frequency HFOs in epileptic rats. Ripple frequency oscillations, therefore, could be either normal or pathologic, and there is no easy way to distinguish between these 2 types of ripples on EEG.11, 12 Furthermore, HFOs in the fast ripple range can occur in primary areas of neocortex.11, 12
In addition to a large literature associating the presence of pHFOs with epileptogenicity in animal models of epilepsy, and the fact that removal of pHFO‐generating brain areas is associated with a seizure‐free surgical outcome in patients,2, 3 clinical studies indicate that pHFOs are biomarkers of epileptogenicity regardless of lesion type,13 and measure the degree of epileptogenicity.14 In the latter study, whereas interictal spikes increase postictally when seizure threshold is high, pHFOs do not increase, but they do increase after medication reduction when seizure threshold is reduced, demonstrating that pHFOs more reliably indicate the degree of epileptogenicity than interictal spikes.14 Animal studies have demonstrated that pHFOs occur early after 2 epileptogenic insults, intrahippocampal kainic acid injection15 and fluid percussion TBI,16 and reliably predict animals that will go on to have spontaneous seizures. pHFOs have yet to be confirmed as reliable biomarkers of epileptogenesis in patients.
Although pHFOs currently are the most promising epilepsy biomarkers for clinical application, a major limitation is that most of the animal and human work that has been done to demonstrate their reliability in specific situations requires direct brain recording. Initial studies were carried out with microelectrodes best capable of identifying activity in local microcircuits,7, 8, 17 but more recent work has clearly demonstrated the ability of clinical depth and subdural electrodes to identify both ripple and fast ripple frequency HFOs.18, 19, 20, 21 Of interest, with the larger clinical electrodes, ripple frequency HFOs have almost the same ability to localize the epileptogenic region as fast ripple frequency HFOs, perhaps due to the more localized dipoles of pathologic events, compared to the more distributed dipoles of normal events.12
Given that pHFOs have largely been identified by direct brain recording, the majority of studies confirming their reliability as a biomarker of epilepsy have been limited to identification of the epileptogenic region for surgery, which is discussed in detail in the next chapter (J. Wu this issue). Various aspects of HFOs, such as their occurrence on spikes and relationship to slow waves, have been used to distinguish pHFOs from normal HFOs, and novel approaches are utilized to identify false HFOs, and to improve automatic detection.22, 23, 24, 25, 26, 27 Most importantly, however, for the future clinical application of pHFOs to diagnosis and treatment, and to predict patients at risk for epilepsy, work is being done to identify pHFOs noninvasively. Several studies have now reported the ability to record activity in the HFO frequency range from scalp electrodes,28, 29, 30 but it is unclear whether these events are the same as those recorded directly from the brain. Others have reported the ability to record HFOs with magnetoencephalography (MEG).31, 32 It is conceivable that interictal spikes associated with pHFOs will have a metabolic profile on functional magnetic resonance imaging (fMRI) that distinguishes them from interictal spikes without pHFOs, and some data are accumulating suggesting that fMRI may help to identify pHFOs noninvasively.33 Although we are close to accepting that pHFOs, recorded during chronic invasive monitoring, and intraoperatively, delineate the epileptogenic region for surgery, the clinical application of these EEG events for seizure diagnosis, treatment, and prediction, in the routine population of people with epilepsy, remains a hope for the future.
Neuronal Mechanisms of Normal and Pathologic HFOs
Several reviews have been published on mechanisms of normal and pathologic HFOs.34, 35, 36, 37 Here we summarize widely accepted views. Hippocampal ripples are summated IPSPs and reflect the most synchronous physiologic patterns in the mammalian brain.10, 35 They are associated with increased discharges of both pyramidal cells and interneurons.38, 39 Under normal conditions the interneuronal network maintains synchrony of discharges of principal cells at a level below that required for the formation of population spikes. We can make a similar assumption for the mechanism of generation and function of physiologic HFO generation in other brain areas outside of hippocampus.40, 41, 42, 43, 44, 45, 46
pHFOs have a different mechanism of generation. They reflect bursts of population spikes47, 48, 49, 50, 51, 52 in local clusters of principal neurons imbedded in normal tissue.49 A separation of normal and pHFOs based on frequency, as initially was proposed7, 8 is not correct. In rats with epilepsy, abnormal burst of population spikes may occur in the ripple frequency band50 and some pHFOs are a mixture of oscillations in the ripple and fast ripple frequency band,53 whereas in primary neocortical areas oscillations in the fast ripple frequency range can be physiologic.11
The mechanisms generating bursts of population spikes are not known, but studies suggest morphologic alterations associated with lesions are involved in their generation.37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 In rat models of temporal lobe epilepsy, pHFOs occur in hippocampal areas with mossy fiber sprouting,8, 55 and pHFO power increases as tissue cell density decreases.56 Studies in patients with hippocampal sclerosis indicate higher rates of pHFO occurrence and a greater density of pHFO‐generating sites correspond with the severity of cell loss and synaptic reorganization.57, 58 However, the structural disturbances involved in the generation of pHFOs are not consistent,59, 60 and some morphologic alterations appear more epileptogenic than others.13, 61 Microcircuit alterations, such as deficits in astrocyte‐mediated glutamate and γ‐aminobutyric acid (GABA) regulation62, 63, 64 could contribute to neuronal hyperexcitability found in epileptogenic tissue.65, 66 Computer models that combine reduced inhibition, increased “synaptic noise,” which could derive from abnormal neuronal spike firing, and synaptic reorganization can generate pHFOs.67
Mechanism of Ictogenesis
A critical aspect of pHFO pathophysiology is that these unique EEG events, as recorded with microelectrodes in hippocampus, are not generated diffusely in a homogeneous fashion throughout the structure, but, rather, by small clusters of presumably pathologically interconnected neurons (PIN clusters) distributed widely within more normal networks of nonepileptogenic neurons.68 These PIN clusters are spatially stable over time,68 but with reduction in local tonic inhibitory influences, they can increase in size.50 This presents a putative mechanism for ictogenesis11: as a result of a variety of influences that can reduce inhibitory tone within the hippocampus, PIN clusters can increase in size, coalesce, and synchronize until a critical mass is reached and epileptiform discharges are propagated. Animal studies have, indeed, shown that pHFO bursts increase in amplitude and duration prior to ictal events, consistent with this hypothesis.69 Furthermore, in a rat model of mesial temporal lobe epilepsy, seizure frequency is positively correlated with the density of PIN clusters, which is also consistent with this hypothesis.68 Consequently, pHFOs are of interest not only because they are likely biomarkers of a variety of aspects of epilepsy, but because the underlying mechanisms of their generation will likely provide insight into fundamental neuronal mechanisms of epileptogenesis and ictogenesis, and novel targets for antiseizure and antiepileptogenic interventions.
Other Potential EEG Biomarkers
Reports from the animal literature have suggested 3 additional changes in routine noninvasive EEG that appear to correlate with epileptogenesis or ictogenesis. In the fluid percussion TBI rat model, arcuate‐shaped discharges can be recorded with skull screws adjacent to the injury, which appear to predict which animals will eventually develop PTE.16 Microelectrode recordings demonstrate that these arcuate events reflect the presence of pHFOs (Fig. 2). In another study of epileptogenesis in 5 different models of postinjury epilepsy, there was a consistent reduction in theta activity over time, following injury, which predicted the later development of epilepsy with a sensitivity and specificity of 90%.70 Finally, in the rat fluid percussion model of PTE following TBI, 93% of spontaneous seizures occurred during the transition from stage III to rapid eye movement sleep, and a reduction in spindle duration during this time was a specific and sensitive noninvasive biomarker of ictogenesis.71 Scalp EEG is a readily available clinical diagnostic test. If any of these observations in animal models of epilepsy correlate with similar changes in patients, there is a reasonable possibility that specific EEG changes could eventually provide reliable nonictal biomarkers for a variety of aspects of epileptogenesis and epileptogenicity that could profoundly influence diagnosis, treatment, prediction, and prevention.
Figure 2.
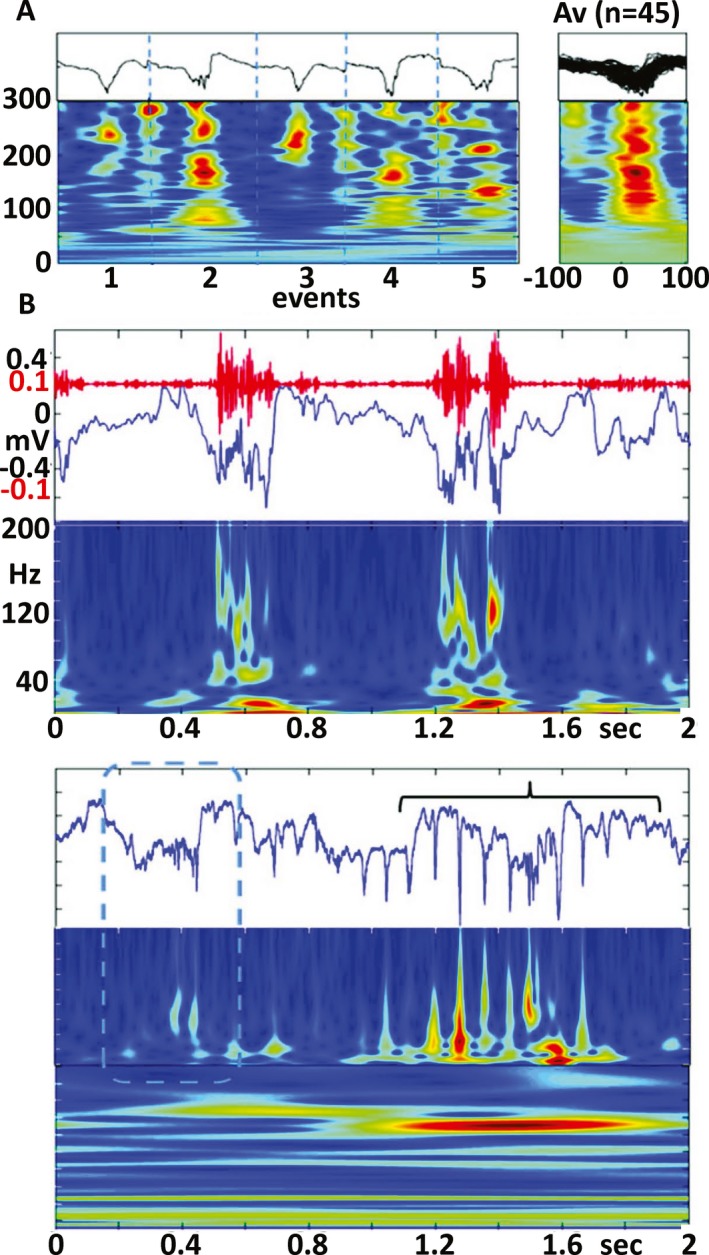
A, Pathologic high frequency oscillations (pHFOs) in the neocortex 3 days after fluid percussion injury. A. Five individual examples (black lines) and time frequency plots (below). (Right) time frequency plots of 45 pHFOs. B, An example of pHFOs associated with a local slow‐wave. C, HFO (dashed box) followed by rHFOs (bracket) containing popSpikes. From Bragin et al16, with permission.
Conclusions
To date, EEG has yielded the most promising nonictal biomarkers of epileptogenesis and epileptogenicity, chief among these being pHFOs. For biomarkers to be clinically useful in diagnosing epilepsy, identifying persons at risk of developing epilepsy after a potential epileptogenic insult or with genetic predisposition, or assessing the effects of antiseizure treatment or antiepileptogenic intervention, it will be necessary to identify them noninvasively.
Efforts, therefore, are now focused on identifying pHFOs with scalp EEG and MEG, as well as validating other potential EEG biomarkers such as arcuate patterns that reflect repetitive pHFOs, changes in theta rhythm, and shortened sleep spindles.
Disclosures
The authors have nothing to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
Original research reported by the authors was supported by grants NS002808, NS015654, NS033310, NS042372, NS080181, NS100064, NS080181, R01NS065877, and U54NS100064 from the National Institutes of Health.
Biography
Dr. Engel is Director of the UCLA Seizure Disorder Center.

References
- 1. Engel J Jr. Seizures and epilepsy. Oxford: Oxford University Press; 2013. [Google Scholar]
- 2. Engel J Jr, Pitkanen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia 2013;54(Suppl. 4):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engel J Jr (ed.). Biomarkers in epilepsy. Biomark Med 2011;5:529–664. [DOI] [PubMed] [Google Scholar]
- 4. Jozwiak S, Becker A, Cepeda C, et al. WONOEP appraisal: development of epilepsy biomarkers – what we can learn from our patients? Epilepsia 2017;58:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pitkanen A, Engel J Jr. Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 2014;11:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engel J Jr. Overview of surgical treatment for epilepsy In Shorvon S, Perucca E, Engel J., Jr (Eds) The treatment of epilepsy. 4th Ed London: Wiley‐Blackwell; 2015:709–722. [Google Scholar]
- 7. Bragin A, Engel J Jr, Wilson CL, et al. High‐frequency oscillations in human brain. Hippocampus 1999;9:137–142. [DOI] [PubMed] [Google Scholar]
- 8. Bragin A, Engel J Jr, Wilson CL, et al. Hippocampal and entorhinal cortex high‐ frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid‐treated rats with chronic seizures. Epilepsia 1999;40:127–137. [DOI] [PubMed] [Google Scholar]
- 9. Bragin A, Engel J Jr, Wilson CL, et al. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia 1999;40:1210–1221. [DOI] [PubMed] [Google Scholar]
- 10. Buzsaki G, Horvath Z, Urioste R, et al. High frequency network oscillation in the hippocampus. Science 1992;256:1025–1027. [DOI] [PubMed] [Google Scholar]
- 11. Engel J Jr, Bragin A, Staba R, et al. High‐frequency oscillations: what is normal and what is not? Epilepsia 2009;50:598–604. [DOI] [PubMed] [Google Scholar]
- 12. Engel J Jr, da Silva FL. High‐frequency oscillations – where we are and where we need to go. Prog Neurobiol 2012;98:316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs J, Levan P, Châtillon CE, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 2009;132:1022–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zijlmans M, Jacobs J, Zelmann R, et al. High‐frequency oscillations mirror disease activity in patients with epilepsy. Neurology 2009;72:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bragin A, Staba RJ, Engel J Jr. The significance of interictal fast ripples in the evaluation of the epileptogenic zone In Lüders HO. (Ed) Textbook of epilepsy surgery. London: Taylor and Francis Medical Books; 2008:530–536. [Google Scholar]
- 16. Bragin A, Li L, Almajano J, et al. Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia 2016;57:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bragin A, Staba R, Engel J. The significance of interictal fast ripples in the evaluation of epileptogenic zone In Luders HO. (Ed) Textbook of epilepsy surgery. London: Informa Healthcare; 2008:530–536. [Google Scholar]
- 18. Frauscher B, Bartolomei F, Kobayashi K, et al. High‐frequency oscillations: the state of clinical research. Epilepsia 2017;58:1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobs J, Le Van P, Chander R, et al. Interictal high‐frequency oscillations (80‐500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 2008;49:1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobs J, Zijlmans M, Zelmann R, et al. High‐frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol 2010;67:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Worrell GA, Gardner AB, Stead SM, et al. High‐frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain 2008;131:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roehri N, Pizzo F, Lagarde S, et al. High‐frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann Neurol 2018;83:84–97. [DOI] [PubMed] [Google Scholar]
- 23. Shimamoto S, Waldman ZJ, Orosz I, et al. Utilization of independent component analysis for accurate pathological ripple detection in intracranial EEG recordings recorded extra‐ and intra‐operatively. Clin Neurophysiol 2018;129:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song I, Orosz I, Chervoneva I, et al. Bimodal coupling of ripples and slower oscillations during sleep in patients with focal epilepsy. Epilepsia 2017;58:1972–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Ellenrieder N, Dubeau F, Gotman J, et al. Physiological and pathological high‐ frequency oscillations have distinct sleep‐homeostatic properties. Neuroimage Clin 2017;14:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiss SA, Orosz I, Salamon N, et al. Ripples on spikes show increased phase‐ amplitude coupling in mesial temporal lobe epilepsy seizure‐onset zones. Epilepsia 2016;57:1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zijlmans M, Worrell GA, Dumpelmann M, et al. How to record high‐frequency oscillations in epilepsy: a practical guideline. Epilepsia 2017;58:1305–1315. [DOI] [PubMed] [Google Scholar]
- 28. Andrade‐Valenca LP, Valenca MM, Ribeiro LT, et al. Clinical and neuroimaging features of good and poor seizure control patients with mesial temporal lobe epilepsy and hippocampal atrophy. Epilepsia 2003;44:807–814. [DOI] [PubMed] [Google Scholar]
- 29. Cuello‐Oderiz C, von Ellenrieder N, Dubeau F, et al. Influence of the location and type of epileptogenic lesion on scalp interictal epileptiform discharges and high‐ frequency oscillations. Epilepsia 2017;58:2153–2163. [DOI] [PubMed] [Google Scholar]
- 30. Melani F, Zelmann R, Dubeau F, et al. Occurrence of scalp‐fast oscillations among patients with different spiking rate and their role as epileptogenicity marker. Epilepsy Res 2013;106:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mendell JT, ap Rhys CM, Dietz HC. Separable roles for rent1/hUpf1 in altered splicing and decay of nonsense transcripts. Science 2002;298:419–422. [DOI] [PubMed] [Google Scholar]
- 32. Velmurugan J, Nagarajan SS, Mariyappa N, et al. Magnetoencephalographic imaging of ictal high‐frequency oscillations (80‐200 Hz) in pharmacologically resistant focal epilepsy. Epilepsia 2018;59:190–202. [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez Otarula KA, Khoo HM, von Ellenrieder N, et al. Spike‐related haemodynamic responses overlap with high frequency oscillations in patients with focal epilepsy. Brain 2018;141:731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bragin A, Engel JJ, Staba RJ. High‐frequency oscillations in epileptic brain. Curr Opin Neurol 2010;23:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buzsáki G, da Silva FL. High frequency oscillations in the intact brain. Prog Neurobiol 2012;98:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jefferys JG, Menendez de la Prida L, Wendling F, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol 2012;98:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiruska P, Alvarado‐Rojas C, Schevon CA, et al. Update on the mechanisms and roles of high‐frequency oscillations in seizures and epileptic disorders. Epilepsia 2017;58:1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klausberger T, Magill PJ, Marton LF, et al. Brain‐state‐ and cell‐type‐specific firing of hippocampal interneurons in vivo. Nature 2003;421:844–848. [DOI] [PubMed] [Google Scholar]
- 39. Ylinen A, Bragin A, Nadasdy Z, et al. Sharp wave‐associated high‐frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 1995;15:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chrobak JJ, Buzsaki G. High‐frequency oscillations in the output networks of the hippocampal‐ entorhinal axis of the freely behaving rat. J Neurosci 1996;16:3056–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80‐200 hz) in neocortex and their neuronal correlates. J Neurophysiol 2001;86:1884–1898. [DOI] [PubMed] [Google Scholar]
- 42. Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80‐200 Hz) during seizures: intracellular correlates. J Neurophysiol 2003;89:841–852. [DOI] [PubMed] [Google Scholar]
- 43. Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol 1999;82:1599–1609. [DOI] [PubMed] [Google Scholar]
- 44. Jones MS, MacDonald KD, Choi B, et al. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol 2000;84:1505–1518. [DOI] [PubMed] [Google Scholar]
- 45. Khodagholy D, Gelinas JN, Buzsaki G. Learning‐enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 2017;358:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ponomarenko AA, Korotkova TM, Haas HL. High frequency (200 Hz) oscillations and firing patterns in the basolateral amygdala and dorsal endopiriform nucleus of the behaving rat. Behav Brain Res 2003;141:123–129. [DOI] [PubMed] [Google Scholar]
- 47. Ibarz JM, Foffani G, Cid E, et al. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci 2010;30:16249–16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci 2004;24:8896–8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bragin A, Benassi SK, Kheiri F, et al. Further evidence that pathologic high‐frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia 2011;52:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bragin A, Mody I, Wilson CL, et al. Local generation of fast ripples in epileptic brain. J Neurosci 2002;22:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bragin A, Wilson CL, Engel J. Voltage depth profiles of high‐frequency oscillations after kainic acid‐induced status epilepticus. Epilepsia 2007;48:35–40. [DOI] [PubMed] [Google Scholar]
- 52. Jones RT, Barth AM, Ormiston LD, et al. Evolution of temporal and spectral dynamics of pathologic high‐frequency oscillations (pHFOs) during epileptogenesis. Epilepsia 2015;56:1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bragin A, Mody I, Engel J Jr. Pathological high frequency oscillations reflect hypersynchronization of action potentials. Neuron 2007;55:930–941, comment.17880896 [Google Scholar]
- 54. Menendez de la Prida L, Trevelyan AJ. Cellular mechanisms of high frequency oscillations in epilepsy: on the diverse sources of pathological activities. Epilepsy Res 2011;97:308–317. [DOI] [PubMed] [Google Scholar]
- 55. Bragin A, Wilson CL, Engel J Jr. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia 2000;41:S144–S152. [DOI] [PubMed] [Google Scholar]
- 56. Foffani G, Uzcategui YG, Gal B, et al. Reduced spike‐timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 2007;55:930–941. [DOI] [PubMed] [Google Scholar]
- 57. Ogren JA, Wilson CL, Bragin A, et al. Three‐dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol 2009;66:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia 2007;48:2130–2138. [DOI] [PubMed] [Google Scholar]
- 59. Bragin A, Wilson CL, Almajano J, et al. High‐frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia 2004;45:1017–1023. [DOI] [PubMed] [Google Scholar]
- 60. Jiruska P, Finnerty GT, Powell AD, et al. Epileptic high‐frequency network activity in a model of non‐lesional temporal lobe epilepsy. Brain 2010;133:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferrari‐Marinho T, Perucca P, Mok K, et al. Pathologic substrates of focal epilepsy influence the generation of high‐frequency oscillations. Epilepsia 2015;56:592–598. [DOI] [PubMed] [Google Scholar]
- 62. Campbell SL, Hablitz JJ, Olsen ML. Functional changes in glutamate transporters and astrocyte biophysical properties in a rodent model of focal cortical dysplasia. Front Cell Neurosci 2014;8:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ortinski PI, Dong J, Mungenast A, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 2010;13:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robel S, Buckingham SC, Boni JL, et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 2015;35:3330–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Staba RJ, Ekstrom AD, Suthana NA, et al. Gray matter loss correlates with mesial temporal lobe neuronal hyperexcitability inside the human seizure‐onset zone. Epilepsia 2012;53:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Staba RJ, Wilson CL, Bragin A, et al. Sleep States differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J Neurosci 2002;22:5694–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stacey WC, Krieger A, Litt B. Network recruitment to coherent oscillations in a hippocampal computer model. J Neurophysiol 2011;105:1464–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bragin A, Wilson CL, Engel J. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia 2003;44:1233–1237. [DOI] [PubMed] [Google Scholar]
- 69. Bragin A, Azizyan A, Almajano J, et al. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia 2005;46:1592–1598. [DOI] [PubMed] [Google Scholar]
- 70. Milikovsky DZ, Weissberg I, Kamintsky L, et al. Electrocorticographic dynamics as a novel biomarker in five models of epileptogenesis. J Neurosci 2017;37:4450–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Andrade P, Nissinen J, Pitkanen A. Generalized seizures after experimental traumatic brain injury occur at the transition from slow‐wave to rapid eye movement sleep. J Neurotrauma 2017;34:1482–1487. [DOI] [PubMed] [Google Scholar]


