Figure 1.
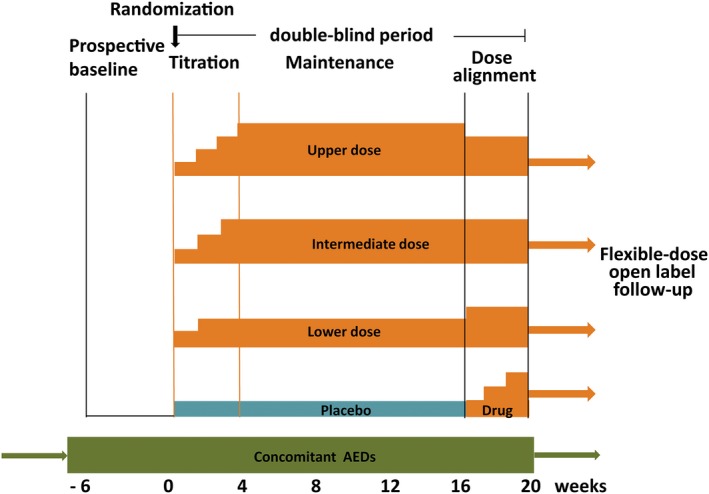
Representative double‐blind trial design for assessing the efficacy and tolerability of investigational new antiepileptic drugs (AEDs) given as adjunctive therapy. After an initial 4‐ to 8‐week prospective evaluation to establish a baseline, patients are randomized to 3 parallel‐dose groups or placebo. Treatment generally includes a titration period of variable length and modalities, and a 12‐week maintenance period. Efficacy is evaluated by comparing changes in seizure frequency or responder rate (versus baseline) between each dose group and the placebo group. The treatment period used for efficacy assessment typically includes the titration and maintenance period combined (FDA‐preferred analysis) or the maintenance period alone (EMA‐preferred analysis). In the trial design illustrated in the figure, the maintenance phase is followed by a dose‐alignment phase during which all patients are blindly converted to a common dose in order to preserve the double‐blind. When dose alignment is completed, open‐label flexible‐dose treatment can be continued long‐term as clinically indicated. The dose alignment phase also allows patients initially exposed to placebo to receive a trial treatment with the investigational drug.
