Key Clinical Message
Acute generalized exanthematous pustulosis (AGEP) is a self‐limited drug reaction. Hydroxychloroquine (HCQ) is an uncommon cause of AGEP with a prolonged recovery course; Thus, the physicians should take the possibility of this rare but severe event in their minds and try to diagnose correctly and better management.
Keywords: acute generalized exanthematous pustulosis, hydroxychloroquine
1. INTRODUCTION
Acute generalized exanthematous pustulosis (AGEP) is a rare cutaneous eruption that often induced by drugs and >90% antibiotics (mainly beta lactams) are most frequent triggers.1
Acute generalized exanthematous pustulosis is characterized by acute onset of wide spread nonfollicular pinpoint aseptic pustules overlying erythematous skin. This cutaneous eruption often accompanied by fever (38°C <), leukocytosis and spontaneous resolution within < 15 days that typically followed by desquamation. Frequent pathological features include sub/intracorneal pustules contain neutrophils and eosinophils, necrotic keratinocytes, papillary edema, spongiosis, dermal neutrophilic infiltration with eosinophils and generally absence of vasculitis.3 No specific treatment is available. Although in more severe and prolonged cases, systemic corticosteroids are usually administered.4, 5 Although the pathogenesis of AGEP is not yet known, a role of drug‐specific T cell has recently been proposed.6, 7
Hydroxychloroquine (HCQ) is an antimalarial drug that has been widely used in dermatologic and rheumatologic diseases and is reported as a rare cause of AGEP.
We presented a case of prolonged HCQ‐ induced AGEP that the eruption waxes and wanes and cleared after gradually tapering of corticosteroid within 68 days.
We also reviewed the literature in English from 2008 to 2018 and found nine other cases that developed AGEP secondary to HCQ.7, 8, 9, 10, 11, 12, 13
2. CASE REPORT
A 44‐year‐old white woman with a 5‐month history of distal joint pain was started on HCQ 200 mg daily. Five days after initiation of HCQ, the patient developed pruritic erythematous patches with pustules on upper chest and upper limbs.
Despite topical steroids, the lesions persisted and deteriorated. She visited her primary rheumatologist after 10 days, and she presented to rheumatology clinic. HCQ was immediately withdrawn after 10 days. She was started on 30 mg prednisolone daily and was visited by the dermatologist. Skin biopsy, stopping of HCQ, and supportive treatment including antihistamines, topical steroids, and intravenous hydration were planned for her.
Skin biopsy demonstrated nonfollicular Superficial pustules in the epidermis filled with neutrophils, a mixed eosinophilic and neutrophilic perivascular infiltration and absence of psoriasis‐like changes that consisted with AGEP (Figure 1).
Figure 1.
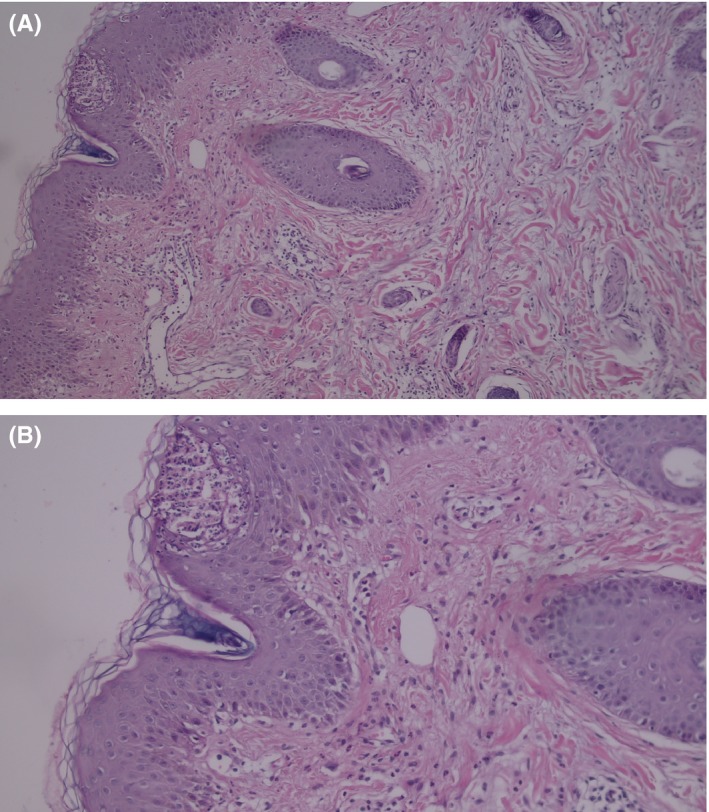
Superficial intraepidermal pustules and perivascular and interstitial mixed infiltrate containing neutrophils, lymphocytes, and some eosinophils in the upper dermis. Hematoxylin and eosin, original magnification : (A) ×40, (B) ×100
After moderately controlled the lesions, the patient was discharged and 30 mg prednisolone was planned to taper gradually by 5‐10 mg weekly, 2 weeks later when the patient was treated on 20 mg prednisolone, once daily she attended the dermatology clinic.
She developed a wide pustular exanthema on her trunk and limbs that gradually spread on the face and scalp. Some annular erythematous lesions and erythematous patches with targetoid appearance with scale and studded nonfollicular pinpoint pustules were also seen on her legs (Figure 2).
Figure 2.
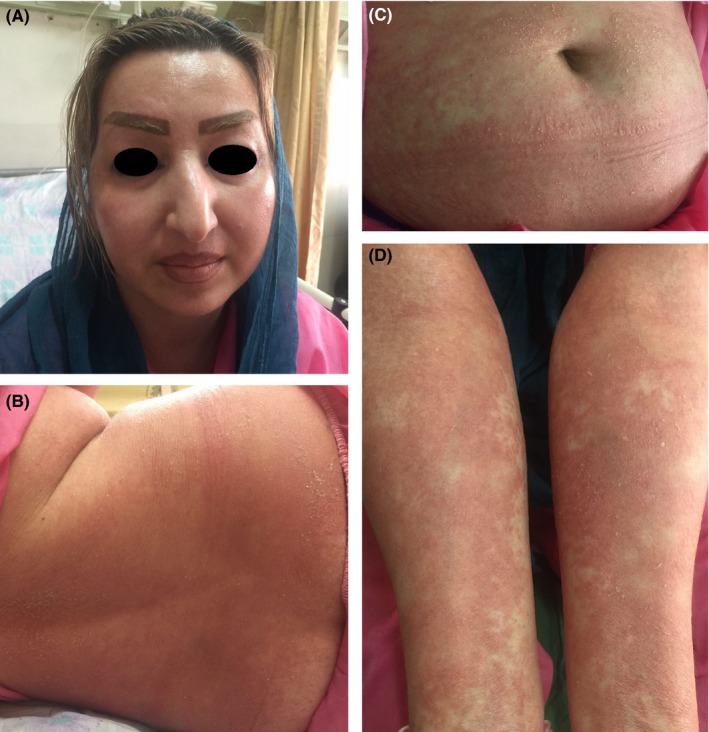
Facial involvement (A) small nonfollicular pustules on the back, abdomen and forearms on erythematous skin with typical extensive postpustular desquamation (B‐D)
Mucosal membrane, nail, and palmoplantar surface were spared.
She described the chills, lethargy, painful stinging, and pruritus sensation as the lesions spread. The patient had no personal/familial history of psoriasis.
She has a history of fever with a temperature of 38.7°C, but on admission, the vital signs were stable.
She had a high white blood cells count with a left shift (WBC 14 700, normal range, 4‐10 × 109/L neutrophil count 10 900 equivalent to 86.1%).
Her septic workup including a CXR, blood culture, urine culture, and other routine laboratory including renal and liver function tests were unremarkable. Punch biopsy and swabs of the pustules were taken.
Bacterial and fungal cultures of the pustules were negative. The results of serologic screening for EBV, CMV, HBV, and mycoplasma were also negative. Later, we were informed that the initiation of hydroxychloroquine was due to a misdiagnosis and arthritis was ruled out by both laboratory and radiological investigations and her comfort was more related to arthralgia.
She was started topical steroids three times daily, antihistamine and oral prednisolone at a dosage of 35 mg daily. On second day, the eruption continued to persist and she reported development of new pustules and worsening of pain and pruritus. Antihistamine dosage was elevated and because of pathological demonstration of AGEP and rule out of psoriasis, prednisolone dosage was switched to 50 mg daily on fourth day.
After 15 days in hospital, the pustules and erythema were moderately improved and she had wide spread desquamations. She was discharged on tapering doses of oral prednisolone 50 mg/daily, antihistamine, and topical steroids twice daily. The patient visited 37 days after discharge with mild pruritic eruption and desquamation on upper limbs and trunk and distal lower extremities. She was instructed to taper prednisolone 5 mg weekly.
At a follow‐up 3 months later, the eruption was completely resolved with no recurrence and the systemic corticosteroid was tapered totally. She was also referred to a psychologist because of intermittent complaint about joint pain.
3. DISCUSSION
Acute generalized exanthematous pustulosis typically presents as a pustular rash with a diffuse often with acute onset, edematous that arises predominately intertriginous areas or on the face. Edema of the face/purpura, vesicle, blister, erythema multiform‐like lesions, and mucosal membrane involvement has also been described. Mild eosinophilia, transient renal failure, hypocalcemia, and elevated amino transferase levels may accompany fever and neutrophilic leukocytosis.14 Lymphadenopathy has described in some cases.15 Once the causative drugs have been discontinued, spontaneous resolution within 15 days occurs.2
Due to the benign self‐limiting course of AGEP, specific treatment usually is unnecessary in mild cases. Systemic corticosteroids are usually used for severe cases.4, 5, 7 Some authors have reported using dapsone and cyclosporine in treatment of severe and atypical forms of AGEP as case reports.9, 13
The pathogenesis of AGEP is not yet known although release of increased amount of IL8 by T cells with attraction and activation of polymorphous nuclear neutrophils has recently been proposed. And the disease is associated with human leukocyte antigens B51, DR11, and DQ3.7
Histologically, AGEP is composed by nonfollicular spongiotic pustules in the epidermis filled with neutrophils, papillary edema, and perivascular infiltration of neutrophils and associated eosinophilia.3
Differential diagnosis of AGEP includes bacterial folliculitis, varicella, impetiginized, eczema, staphylococcal‐scalded skin syndrome, and pustular psoriasis, that is, serologically, clinically, and histologically distinguishable. Distinguish between AGEP and generalized pustular psoriasis specially von Zumbusch‐type is important; recent drug administration, clinical course, and histopathological features help to distinguish between them. The pustules of pustular psoriasis are usually larger and show more prominent spongiform postulations, whereas necrotic keratinocytes, edema of papillary dermis, presence of eosinophils, mixed eosinophilic and neutrophilic infiltrations both in pustules and dermis, extravasation of erythrocytes, leukocytoclasia (rarely vasculitis), and absences of both tortuous blood vessels and significant epidermal psoriasiform changes are more prominent in AGEP.3, 16, 17
There are some reports of developing AGEP in patients with psoriasis, but no significant differences were observed between them and the patients with no history of psoriasis.3, 7
In AGEP, the average duration of drug exposure prior to onset of the symptom depends on the causative drug. Antibiotic consistently have been shown to trigger AGEP after 1 day, whereas often medication, including HCQ, averaged closer to 11 days.18
Hydroxychloroquine is an antimalarial medication with a half‐life of 1‐2 months, that is, a lysosomotrophic amin entering the lysosome of antigen‐presenting cells and raising the PH. HCQ is also an anti‐inflammatory agent and is thought to act by blocking the activities of toll‐like receptors on plasmocytoid dendritic cells. These features have to lead its use in the treatment of rheumatologic and dermatologic conditions.19, 20
Hydroxychloroquine has been described as a rare cause of AGEP. In a literature search by Paradisi and colleagues over 14 years between 1993 and 2007, only 16 report HCQ‐induced AGEP were reported.7
We also reviewed the literature in English since 2008 to 2018 and found nine other cases.7, 8, 9, 10, 11, 12, 13 The details of the cases are summarized in Table 1. The cases have been cleared within 8‐91 days. And our patient showed resolution after 68 days.
Table 1.
Description of nine cases of hydroxychloroquine (HCQ)‐induced AGEP
| Age/sex | HCQ dosage | Time of onset | Treatment | Resolution of AGEP | |
|---|---|---|---|---|---|
| Paradise et al7 (2008) | 36/F | 100 mg BID | 21 d |
Prednisolone 40 mg/d reduced By 10 mg every 4 d |
8 d |
| 70/M |
100 mg BD DC in 8 d |
20 d | Prednisolone 40 mg/d | 12 d | |
| 79/M |
100 mg BD D/C in 2 d |
20 d | Prednisolone 40 mg/d | 15 d | |
| Lateef et al8 (2009) | 67/F | Not reported | 3 wk | Supportive for AGPE corticosteroid and IVIG for TEN | Hospitalized 2 W |
| Di Lernia et al9 (2009) | 63/F |
100 mg (BD) D/C 7 d Into rash |
30 d | Cyclosporine | Exacerbation at 18 d, tapered corticosteroids |
| Park et al10 (2010) | 38/F |
200 mg D/C 7 d Into rash |
3 wk | Methyl prednisolone 125 mg | Not reported |
| Bailey et al11 (2013) | 48/F | 200 mg HIQ | 14 d | Methyl prednisolone prednisolone | 18 d |
| Pearson et al12 (2016) | 50/F | 200 mg BD | 2 wk | Methyl prednisolone 100 mg/daily | Exacerbation at 1th wk and 3th wk |
| Duman et al13 (2017) | 42/F | 200 mg daily | 21 d |
Methyl prednisolone 60 mg/daily Dapsone 50 mg/daily |
35 d |
These cases have history of initiation of HCQ within 2‐3 weeks prior to developing the symptoms, whereas in our patient, the eruption initially arose within 5 days.
4. CONCLUSION
Acute generalized exanthematous pustulosis is a drug‐induced pattern that often has a spontaneous resolution. HCQ is used extensively in rheumatologic and dermatologic conditions and is a rare cause of AGEP. Severe and prolonged cases of HCQ‐induced AGEP have been reported. Cessation is generally effective for therapy, but treatment is sometimes difficult. Thus, the physicians should take the possibility of this rare but severe event in their minds.
Recurrent and prolonged AGEP secondary to HCQ may be due to long half‐life of HCQ which is approximately 40‐50 days.
Utility of systemic corticosteroids in shortening the duration or decreasing the severity of the reaction has not been proven yet. Also efficacy of other drugs like dapsone or cyclosporine in this condition has not studied yet.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
MJ and FM: provided medical care, reviewed the medical records, interpreted data, and drafted the manuscript. FM and PR: provided pathological photographs and interpreted pathological data.
Mohaghegh F, Jelvan M, Rajabi P. A case of prolonged generalized exanthematous pustulosis caused by hydroxychloroquine—Literature review. Clin Case Rep. 2018;6:2391–2395. 10.1002/ccr3.1811
REFERENCES
- 1. Roujeau JC, Bioulac‐Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333‐1338. [PubMed] [Google Scholar]
- 2. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP) – a clinical reaction pattern. J Cutan Pathol. 2001;28:113‐119. [DOI] [PubMed] [Google Scholar]
- 3. Halevy S, Kardaun SH, Davidovici B, Wechsler J, EuroSCAR and RegiSCAR Study Group . The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010;163(6):1245‐1252. [DOI] [PubMed] [Google Scholar]
- 4. Lotem M, Ingber A, Segal R, Sandbank M. Generalized pustular drug rash induced by hydroxychloroquine. Acta Derm Venereol. 1990;70:250‐251. [PubMed] [Google Scholar]
- 5. Martins A, Lopes LC, Paiva Lopes MJ, Rodrigues JC. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Eur Dermatol. 2006;16:317‐318. [PubMed] [Google Scholar]
- 6. Girardi M, Duncan KO, Tigelaar RE, et al. Cross‐comparison of patch test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343‐346. [DOI] [PubMed] [Google Scholar]
- 7. Paradisi A, Bugatti L, Sisto T, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008;30:930‐940. [DOI] [PubMed] [Google Scholar]
- 8. Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine. Clin Rheumatol. 2009;28:1449‐1452. [DOI] [PubMed] [Google Scholar]
- 9. Di Lernia V, Grenzi L, Guareschi E, et al. Rapid clearing of acute generalized exanthematous pustulosis after administration of cyclosporine. Clin Exp Dermatol. 2009;34:e757‐e759. [published online July 29, 2009]. [DOI] [PubMed] [Google Scholar]
- 10. Park JJ, Yun SJ, Lee JB, et al. A case of hydroxychloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation. Ann Dermatol. 2010;22:102‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailey K, Mckee D, Wismer J, Shear N. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: first case report in Canada and review of the literature Canadian Dermatology Association. J Cutan Med Surg. 2013;17(6):414‐418. [DOI] [PubMed] [Google Scholar]
- 12. Pearson KC, Morrell DS, Runge SR, Jolly P. Prolonged pustular eruption from hydroxychloroquine: an unusual case of acute generalized exanthematous pustulosis. Cutis. 2016;97(3):212‐216. [PubMed] [Google Scholar]
- 13. Duman H, Topal IO, Kocaturk E, Cure K, Mansuroglu I. Acute generalized exanthematous pustulosis induced by hydroxychloroquine:a case with atypical clinical presentation. An Bras Dermatol. 2017;92(3):404‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valeyrie‐Allanore L, Obied G, Revuz J. Drug Reactions In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology, 4th edn New York City, NY: ELSEVIER; 2018:348‐375. [Google Scholar]
- 15. Eeckhout I, Noens L, Ongenae K, et al. Acute generalized exanthematic pustulosis: a case with a lymphoma like presentation. Dermatology. 1997;194:408‐410. [DOI] [PubMed] [Google Scholar]
- 16. Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasis. J Cutan Pathol. 2010;37(12):1220‐1229. [DOI] [PubMed] [Google Scholar]
- 17. Patterson JW. The Vesiculobullous Reaction Pattern. Weldon's Skin Pathology, 4th edn New York City, NY: Elsevier; 2015. [Google Scholar]
- 18. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exhanthematous pustulosis(AGEP)—results of a multinational case‐control study (EuroSCAR). Br J Dermatol. 2007;157:989‐996. [DOI] [PubMed] [Google Scholar]
- 19. Mackenzie AH. Pharmacologic actions of 4‐aminoquinoline compounds. Am J Med. 1983;75:5‐10. [DOI] [PubMed] [Google Scholar]
- 20. Wallace DJ. The history of antimalarials. Lupus. 1966;5(Suppl 1):S2‐S3. [PubMed] [Google Scholar]


