Fig. 2.
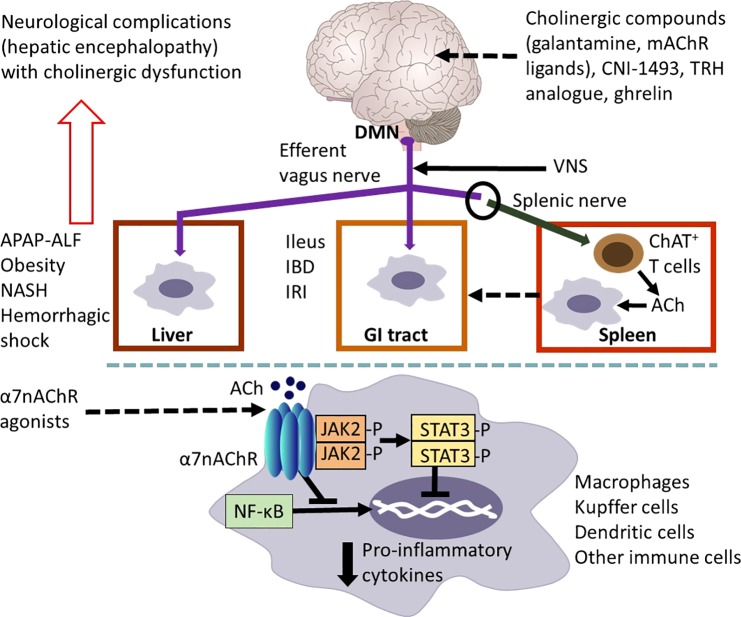
Vagus nerve cholinergic signaling regulates inflammation in the gastrointestinal (GI) tract and the liver. Efferent vagus nerve preganglionic cholinergic fibers (shown in purple) originating predominantly from the dorsal motor nucleus of the vagus (DMN) innervate the liver and the GI tract and regulate local immune responses, inflammation, and metabolism. The use of vagotomy, vagus nerve stimulation (VNS), knockout mice, and the administration of cholinergic compounds, including α7 nicotinic receptor (α7nAChR) agonists have been instrumental in identifying the functional anatomy and molecular components of this regulation. Cholinergic signaling has been implicated in suppressing hepatic inflammation and alleviating metabolic derangements in the context of acetaminophen (APAP)-induced liver injury and acute liver failure (ALF), obesity, nonalcoholic steatohepatitis (NASH) and hemorrhagic shock. Cholinergic signals also control GI inflammation and metabolic disease manifestations in experimental postoperative ileus, inflammatory bowel disease (IBD), and ischemia and reperfusion injury (IRI). A functional cooperation between the efferent vagus nerve and the splenic nerve with the release of acetylcholine (ACh) from a subset of T cells that contain the enzyme choline acetyltransferase (ChAT+ T cells) plays a major mediating role in the inflammatory reflex. Activation of this regulatory circuitry triggered by the central (in the brain) action of the acetylcholinesterase inhibitor, galantamine, and muscarinic ACh receptor (mAChR) ligands plays a role in suppressing colonic inflammation and alleviating the severity of the disease in murine IBD (colitis). Other compounds, including CNI-1493, a stable thyrotropin-releasing hormone (TRH) analog and ghrelin trigger activation of vagus nerve cholinergic signaling that controls immune responses and suppresses inflammation in experimental models of liver and GI disorders. The α7nAChR expressed by macrophages, Kupffer cells, and dendritic cells is a major cellular component of cholinergic regulation of hepatic and GI inflammation. Peripheral cholinergic α7nAChR-mediated activation inhibits NF-κB nuclear translocation, activates JAK2/STAT3 signaling, and suppresses proinflammatory cytokine production. The brain plays an important integrative and regulatory role in the neuroimmune communicatome in the context of liver and GI diseases with documented involvement of cholinergic signaling. However, the brain function can be severely affected (neurological complications known as hepatic encephalopathy) in severe liver diseases with considerable inflammatory involvement. These brain alterations, including cognitive deterioration, are associated with brain cholinergic dysfunction. Findings in preclinical settings have indicated the use of VNS, α7AChR agonists, and centrally acting AChE inhibitors, including galantamine, as therapeutic cholinergic modalities for liver and GI inflammatory disorders. Recent clinical trials with VNS in patients with IBD and with galantamine in patients with the metabolic syndrome have generated promising results [see text for details. The image of the brain has been adapted from Fig. 1, originally published in (43)].