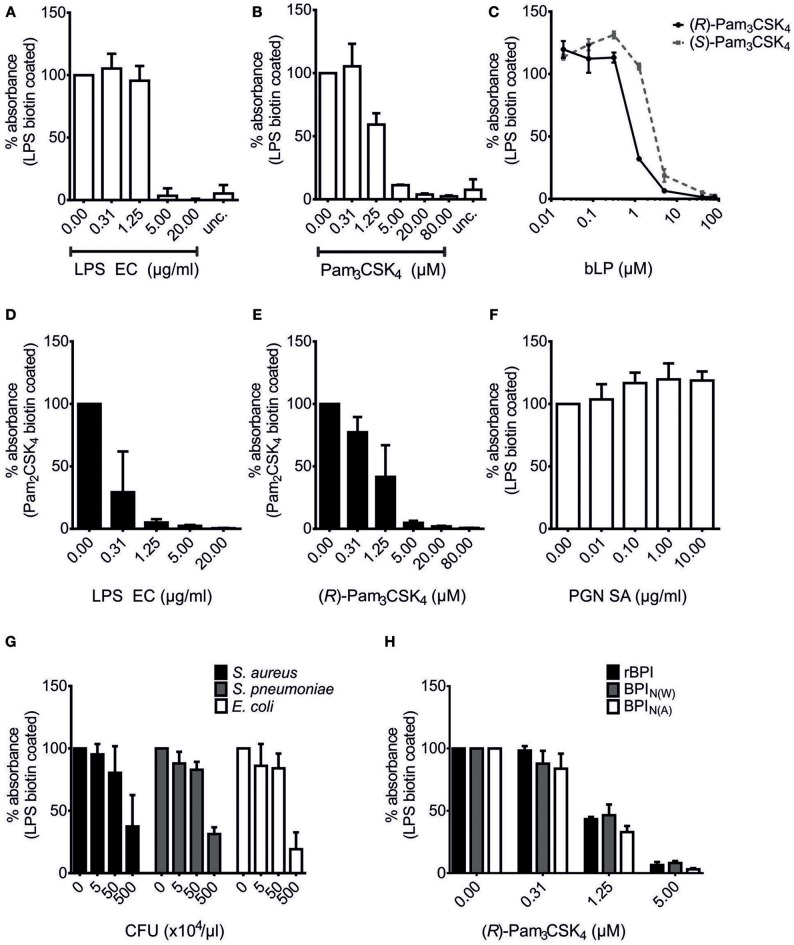
Figure 2.
Competition of bLPs with lipopolysaccharide for binding to BPI. BPI binding assays with LPS biotin (A–C,F–H) or Pam2CSK4 biotin-coated plates (D,E). “unc.” shows binding of rBPI in uncoated wells treated otherwise identically (A,B). rBPI was pre-incubated with increasing concentrations of LPS EC (A,D), the racemate Pam3CSK4 (B), (R)-Pam3CSK4 (C,E) and (S)-Pam3CSK4 (C) or peptidoglycan of S. aureus (PGN SA; F). Furthermore, pre-incubations of rBPI with different heat-inactivated bacterial lysates are shown (G). Preparations of rBPI and neutrophil BPI of two different sources [BPIN(W) and BPIN(A)] were pre-incubated with (R)-Pam3CSK4 (H). Absorbance measured at 450 nm for wells with BPI alone was set to 100% to ensure comparability between the different ligands. All results are shown as means ± SD of three biological replicates.