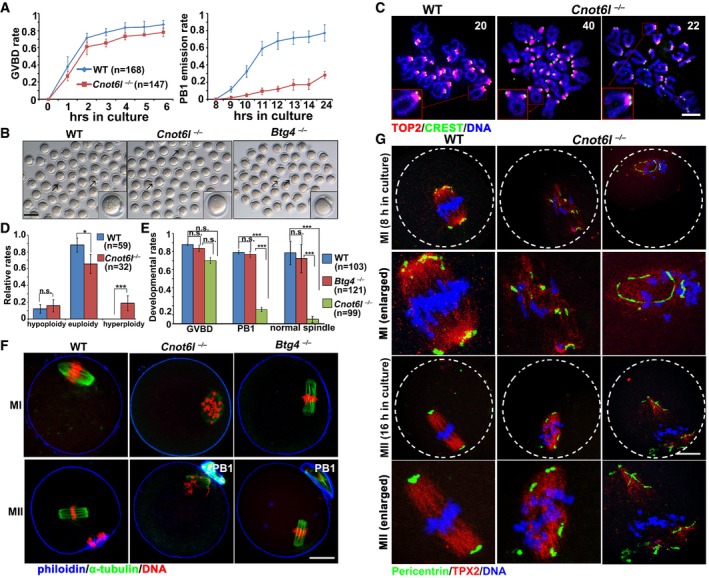
Rates of germinal vesicle breakdown (GVBD) and PB1 emission in oocytes cultured in vitro. Fully grown GV oocytes were collected from PMSG‐primed (44 h) WT and Cnot6l
−/− mice. PB1: polar body‐1. Error bars, SEM. The numbers of analyzed oocytes are indicated (n).
Representative images of WT, Cnot6l
−/−, and Btg4
−/− oocytes showing PB1 emission at 16 h after culture. Arrows indicate PB1. Scale bar, 100 μm.
Representative images of chromosome spreads made from WT and Cnot6l
−/− oocytes after 16 h of in vitro maturation culture. Immunofluorescent staining of topoisomerase II (TOP2) and the centromere antigen CREST were performed to indicate chromosome arms and centromeres, respectively. Numbers of paired sister chromatids are indicated. Scale bar, 5 μm.
Percentage (%) of aneuploidy among in vitro cultured WT and Cnot6l
−/− oocytes that have released PB1s. Error bars, SEM. *P < 0.05; ***P < 0.001 by two‐tailed Student's t‐test. n.s.: non‐significant. The numbers of analyzed oocytes are indicated (n).
Rates of GVBD, PB1 emission, and normal spindle assembly in WT, Cnot6l
−/−, and Btg4
−/− oocytes cultured in vitro. Error bars, SEM. ***P < 0.001 by two‐tailed Student's t‐test. n.s.: non‐significant. The numbers of analyzed oocytes are indicated (n).
Confocal microscopy results showing spindle assembly in WT, Cnot6l
−/−, and Btg4
−/− oocytes at metaphase I (MI) and metaphase II (MII). Scale bar, 20 μm.
Pericentrin immunofluorescence showing MTOCs in cultured WT, Cnot6l
−/−, and Btg4
−/− oocytes at MI and MII stages. Spindle and DNA were labeled by microtubule nucleation factor (TPX2) and DAPI, respectively. Scale bar, 20 μm.