Figure 8. CNOT6L is a downstream effector of ERK1/2 in regulating oocyte meiotic cell cycle progression.
-
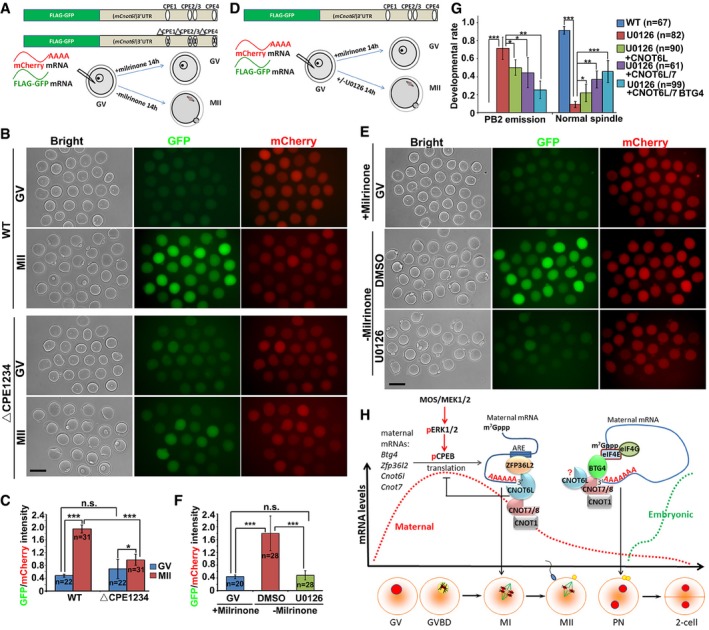
A, BIllustration (A) and fluorescence microscopy results (B) showing the translation activities of the Cnot6l 3′‐UTR (WT and CPE mutated) in GV‐arrested (maintained by 2 μM milrinone) or MII‐arrested (released from milrinone) oocytes. GFP signal indicated translational activation of Cnot6l 3′‐UTR. An in vitro transcribed and polyadenylated mCherry mRNA was co‐microinjected as a positive control. Scale bar, 100 μm.
-
CRelative intensity of GFP signal in (B) after normalization by mCherry signal in the same oocyte. Error bars, SEM. *P < 0.05; ***P < 0.001 by two‐tailed Student's t‐test. n.s.: non‐significant. The numbers of analyzed oocytes are indicated (n).
-
D, EIllustration (D) and fluorescence microscopy results (E) showing the expression of GFP‐fused Cnot6l 3′‐UTR in GV and MII oocytes with or without U0126 treatment (20 μM). Scale bar, 100 μm.
-
FRelative intensity of GFP signal in (E) after normalization by mCherry signal in the same oocyte. Error bars, SEM. ***P < 0.001 by two‐tailed Student's t‐test. n.s.: non‐significant. The numbers of analyzed oocytes are indicated (n).
-
GRates of PB2 emission and normal spindle assembly in oocytes cultured with or without U0126. Fully grown GV oocytes were microinjected with mRNAs encoding CNOT6L, CNOT7, and/or BTG4 and are released from milrinone at 12 h after microinjection. Then, the oocytes were further culture for 24 h with or without adding U0126 to the medium. Error bars, SEM. *P < 0.05; **P < 0.01; ***P < 0.001 by two‐tailed Student's t‐test. n.s.: non‐significant. The numbers of analyzed oocytes are indicated (n).
-
HA diagram showing role of CCR4–NOT deadenylase complex in targeting maternal mRNA decay. During the onset of oocyte meiotic resumption, MAPK cascade and CPEB1 triggers translational activation of maternal transcripts including those encoding BTG4, CNOT7, CNOT6L, and ZFP36L2 (Belloc & Mendez, 2008; Ma et al, 2015; Yu et al, 2016b; Sha et al, 2017). CNOT6L and other CCR4–NOT components are important downstream effectors of ERK1 and ERK2 in the regulation of spindle assembly and meiotic cell cycle progression in oocytes. During oocyte maturation, RNA‐binding protein ZFP36L2 associates with CNOT6L and functions as a CCR4–NOT adaptor in triggering the degradation of ARE‐containing transcripts. At a later stage of MZT, an alternative adaptor BTG4 binds to translation initiation factor eIF4E and thereby recruits the CCR4–NOT complex to the actively translated mRNAs. The stepwise recruitment of different adaptors by different catalytic subunits mediates stage‐specific degradation of maternal mRNAs by the CCR4–NOT deadenylase. The question mark means that the direct involvement of CNOT6L in BTG4‐mediated MZT process remains inconclusive because Cnot6l null oocytes had severe meiosis defects before MZT.
