Figure 7. Effect of pathogenic CDKL5 mutations on CDKL5 activity.
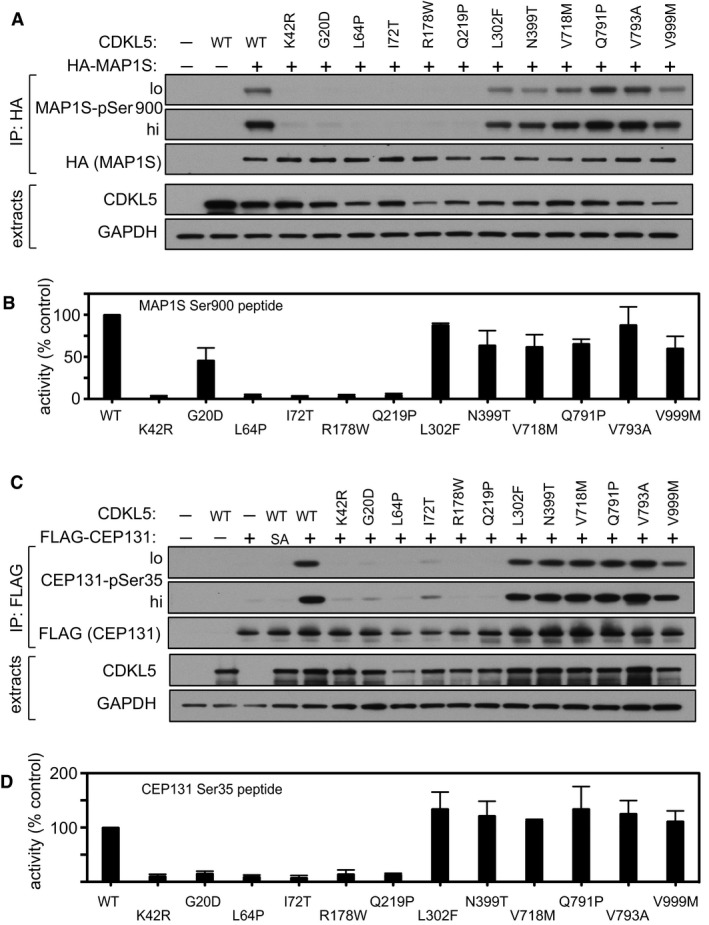
- HEK293 cells were co‐transfected with untagged CDKL5 (wild type “WT” or the mutants indicated) and HA‐tagged MAP1S. Anti‐HA precipitates were subjected to Western blotting with the antibodies indicated. The input extracts were also subjected to immunoblotting (lower panels). Three independent experiments were done, and one representative experiment is shown. Hi, high exposure; lo, low exposure.
- Anti‐FLAG precipitates from HEK293 cells transiently expressing FLAG‐tagged CDKL5 (wild type “WT” or the mutants indicated) were incubated with a synthetic peptide corresponding to the sequence around the MAP1S Ser900 phosphorylation site, in the presence of [γ‐32P]‐labelled ATP‐Mg2+. Peptide phosphorylation was quantitated in a scintillation counter. Data are represented as mean ± SEM from three independent experiments.
- HEK293 cells were co‐transfected with untagged CDKL5 (wild type “WT” or the mutants indicated) and FLAG‐tagged CEP131. Anti‐FLAG precipitates were subjected to Western blotting with the antibodies indicated. The input extracts were also subjected to immunoblotting (lower panels). Three independent experiments were done, and one representative experiment is shown. Hi, high exposure; lo, low exposure.
- Anti‐FLAG precipitates from HEK293 cells transiently expressing FLAG‐tagged CDKL5 (wild type “WT” or the mutants indicated) were incubated with a synthetic peptide corresponding to the sequence around the CEP131 Ser35 phosphorylation site, in the presence of [γ‐32P]‐labelled ATP‐Mg2+. Peptide phosphorylation was quantitated in a scintillation counter. Data are represented as mean ± SEM from three independent experiments.
Source data are available online for this figure.
