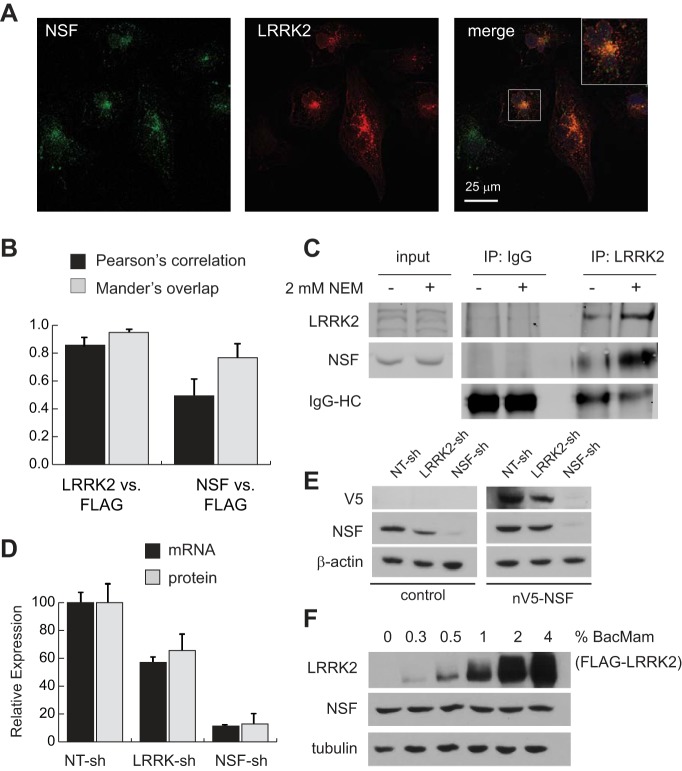
Fig. 4.
LRRK2 and NSF physically and functionally interact in renal epithelia. A: confocal immunofluorescent images of HK2 cells after transfection with FLAG-LRRK2 expression vectors to enable visualization of LRRK2 (red) with endogenous NSF (green). The inset in the merged panel is a ×2.5 magnification of the indicated cell in this image. B: quantification of immunofluorescent signal colocalization between total LRRK2 and the FLAG epitope found on exogenous LRRK2 (positive control), and between NSF and the FLAG epitope using Pearson’s and Mander’s correlation methods. Data represent mean signal overlap from 200 cells for each condition imaged by confocal microscopy. Error bars represent standard deviations of these measurements. C: immunoblot of NSF and LRRK2 after endogenous coimmunoprecipitation with anti-LRRK2 antibodies. Cell lysis buffer with and without 2 mM N-ethylmaleimide (NEM) was used to harvest protein lysates from cells. Input samples represent 10% of the protein input used for immunoprecipitations. D: relative expression of NSF at the mRNA and protein levels in control, LRRK2, or NSF knockdown cell lines. Expression was determined as the ratio of NSF to RPL13A mRNA (qRT-PCR) or β-actin protein (immunoblot) and then normalized to expression in nontargeting control cells (NT-sh). Error bars indicate standard deviations of triplicate samples. E: lysates were harvested from parental HK2 cells (control) and cells stably transfected with V5-tagged human NSF (nV5-NSF) after each line was infected with the indicated lentiviral shRNA vectors. Representative immunoblots demonstrate NSF and V5-tag expression levels relative to actin in cells after introduction of shRNAs. F: immunoblot analysis of lysates from HK2 cells after infection with BacMam/FLAG-LRRK2 at various multiplicities of infection (shown as percent by volume of viral suspension used). Increasing LRRK2 levels has no impact on total levels of NSF protein in cells. Tubulin was used as a loading control to indicate equal loading between samples. LRRK2, leucine-rich repeat kinase 2; HK2, normal human kidney cells; NSF, N-ethylmaleimide-sensitive fusion protein; qRT-PCR, quantitative RT-PCR; shRNA, short hairpin RNA.