Abstract
Here, we present the case of a patient who developed a large hematoma under his tongue while taking concomitant warfarin and ivermectin. Ivermectin has been shown to interfere with vitamin K–dependent clotting factors II, V, VII, and X. However, all the data to date have been from in vivo data with little to no reports on its effects on humans. To our knowledge, this is the first case report to present such a case.
Keywords: adverse drug reactions, anticoagulants, anti-infectives, drug interactions, Warfarin
Background
Numerous warfarin drug interactions are well documented. However, there is a paucity of data that exist on ivermectin utilization in humans, and virtually none with the concomitant use of warfarin. Ivermectin is an antiparasitic medication, primarily used in veterinary medicine, with a broad spectrum of activity but very limited data on utilization in humans.1-5 Ivermectin binds to nerve cells of microfilaria which causes an increase in cell membrane permeability leading to hyperpolarization causing paralysis and cell death.1,2 Ivermectin has been shown, in vitro, to antagonize the same vitamin K–dependent clotting factors that warfarin does (II, VII, IX, and X). Scabies, caused by the mite Sarcoptes scabiei, has been reported by the World Health Organization as a neglected tropical disease, and the treatment options are very limited with ivermectin being an option.1 Patients infected with scabies commonly report itching, rash, and possibly sores. To our knowledge, this is the first case report to discuss an adverse event associated with concomitant warfarin and ivermectin use.
Objective
The objective of this study was to describe a case of ivermectin-induced warfarin toxicity associated with supratherapeutic international normalized ratio (INR) and hematoma development.
Case
A 68-year-old man presented to a critical access emergency department (ED) with a massive hematoma under his tongue which pushed his tongue to the roof of his mouth (Figure 1). His medical history included coronary artery bypass grafting, mechanical aortic and mitral valve replacements, atrial fibrillation, stroke, and scabies. The patient denied alcohol and tobacco use and maintained a relatively strict diet which included minimal foods with a high vitamin K content. Due to his valve replacement and atrial fibrillation, he is chronically anticoagulated with warfarin. After discussion with the patient, it was found he had stable INR checks and no history of supratherapeutic INRs or hematoma development.
Figure 1.
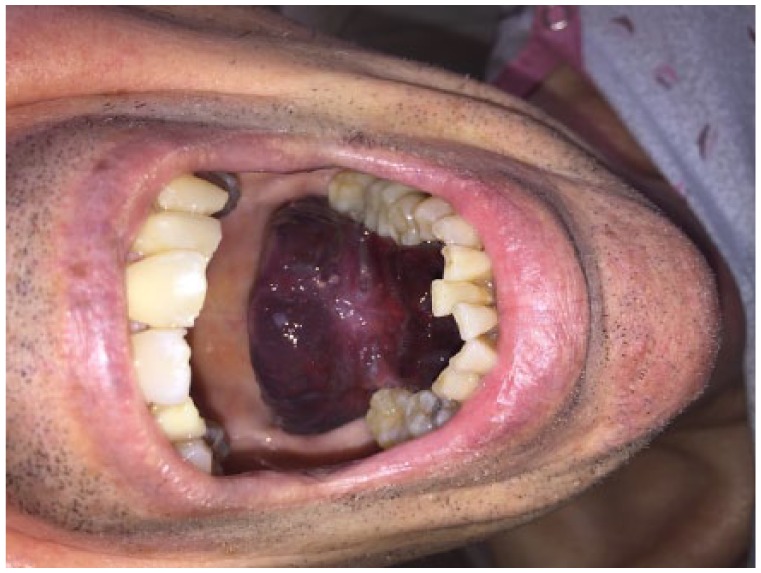
Tongue hematoma.
Prior to his presentation to the outlying ED, he recently had been prescribed 2 doses of ivermectin for suspected scabies infection. The patient was instructed to take 3 mg of ivermectin initially, and repeat 3 mg in 1 month if the scabies symptoms had not resolved. One week prior to the first dose of ivermectin, his INR was 3.1 on warfarin at 5 mg daily. One week after the first dose of ivermectin, his INR had risen to 4.2 for which he was instructed to hold 2 doses and then reinitiate warfarin at 5 mg daily. Per discussion with the patient, his INR decreased back down to 2.7 after 1 week of holding his 2 doses.
One month after his initial ivermectin dose, and 2 days prior to his ED admission, the patient was still experiencing symptoms and therefore, as instructed, took his second 3-mg dose of ivermectin. No other additional medications were taken outside the patient’s chronic therapy. The patient woke the next morning with a large mass under his tongue. He could barely swallow but was not short of air. He proceeded to go to the ED where his INR was found to be >20. He was given 2.5 mg of vitamin K subcutaneously and then transferred to a tertiary care facility due to the complexity of his case. On presentation, the patient’s tongue was pressed up against the roof of his mouth, INR was 20, was inaudible, but still protecting his airway. He was given 10 mg of vitamin K intravenously with a repeat INR 5 hours later of 3.0. At this point, the hematoma under his tongue was decreasing in size but still caused some discomfort to the patient. Due to the subcutaneous injection, the patient also developed an abdominal hematoma (Figure 2). The patient at no point required transfusion, prothrombin complex concentrate, or fresh frozen plasma. He was able to maintain a hemoglobin level of >7 g/dL during his admission. During his admission, the patient had a medication reconciliation performed by the inpatient clinical pharmacy specialist who ruled out any other concomitant medication usage which would interact with warfarin. The Naranjo algorithm was performed on this patient and given a conservative score of 7, which states this reaction followed a reasonable temporal sequence after drug exposure.
Figure 2.
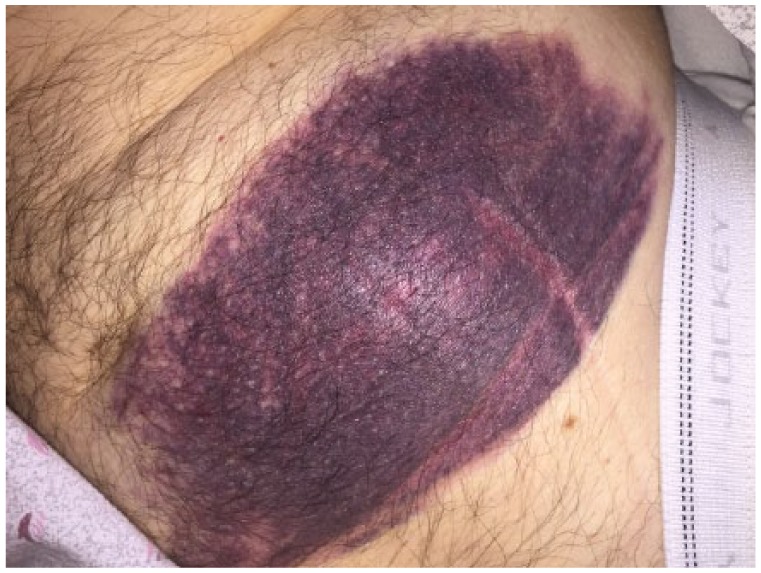
Stomach hematoma.
Discussion
This case exemplifies the importance of thorough medication reviews by prescribers and pharmacists before exposing patients to medications which are not in the usual scope of a clinician’s practice such as in this case the prescribing of an antiparasitic. After oral ingestion of ivermectin, it has been shown to have a half-life of roughly 18 hours but has 10 identified metabolites with unknown activity.2 Ivermectin has been shown to increase prothrombin time by disrupting vitamin K–dependent clotting factors II, V, VII, and X.3-5 In this case, the patient had a positive temporal relationship between the developments of warfarin toxicity induced by ivermectin. While there is a paucity of data that exist on ivermectin utilization in humans, prescribers, pharmacists, and patients must be cognizant of all potential drug-drug interactions that exist with warfarin. There currently do not exist any best practices on the concomitant use of warfarin and ivermectin together; however, based on this case report, prophylactic dose reduction or increased INR monitoring would not be unreasonable. This case report is consistent with the rest of the little literature that exists on ivermectin’s anticoagulant properties by exhibiting prolonged prothrombin time. Although no permanent harm was sustained to our patient, it does highlight the importance of continued monitoring for drug-drug interactions and performance of high-quality medication reviews by prescribers, pharmacists, and patients themselves.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bernigaud C, Fang F, Fischer K, et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl Trop Dis. 2016;10(10):e0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. González Canga A, Sahagún Prieto AM, Diez Liébana MJ, et al. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10(1):42-46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitworth JA, Hay CR, McNicholas AM, Morgan D, Maude GH, Taylor DW. Coagulation abnormalities and ivermectin. Ann Trop Med Parasitol. 1992;86(3):301-305. [DOI] [PubMed] [Google Scholar]
- 4. Hay J, Arnott MA. Ivermectin and coagulation: an in vitro study. Ann Trop Med Parasitol. 1990;84(5):503-506. [DOI] [PubMed] [Google Scholar]
- 5. Richards F, JR, Mcneeley M, Bryan R, et al. Ivermectin and prothrombin time. The Lancet. 1989;333(8647):1139-1140. [PubMed] [Google Scholar]


