Abstract
Background
Age-related neuromuscular changes in the hip abductor-adductor muscles lead to reduced performance, especially in the rate of force development and power production. These alterations may impair weight transfer control and lateral balance recovery through protective stepping. This study compared the effects of eight weeks of low-dose hip abductor-adductor power and strength training on the performance of isometric maximal voluntary contractions, and lateral balance recovery at different initial weight-bearing conditions in older individuals.
Methods
Eighteen healthy older adults (71.3 (0.9)yrs) underwent eight weeks of low-dose hip abductor-adductor exercise training involving either power training (n=10) or lower velocity strength training (n=8). Outcomes were assessed for hip abductor-adductor isometric maximal voluntary contractions and lateral waist-pull balance perturbations with three initial stepping limb-load conditions (50%, 65%, or 80% body mass).
Findings
Power training increased isometric maximal voluntary contractions abductor-adductor peak torque (14%–18%, p<0.05), rate of torque development (31%–39%, p<0.05) and rate of neuromuscular activation (37%–81%, p<0.05). During lateral balance recovery, power training increased the incidence of stabilizing single lateral steps at 80% body mass pre-load (by 43%, p<0.05), reduced step lift-off time by 27ms at 50% body mass (p<0.05) and decreased downward momentum of the body center of mass at 80% body mass (32%, p<0.05). Power training also increased in task hip abductor net joint torque (49%–61%, p<0.05), power (21%–54%, p<0.05), and abductor-adductor rate of neuromuscular activation (17%–62%, p<0.05).
Interpretation
Low-dose hip abductor-adductor power training was more effective than strength training at eliciting improvements in maximal neuromuscular performance and enhanced mediolateral balance recovery.
Keywords: Power training, Lateral Balance, Hip abductors, Neuromuscular performance
1. Introduction
Normal aging is accompanied by an array of changes that occur in the nervous and musculoskeletal systems that lead to a decline in balance and mobility functions and precipitate falls. Age-related neuromuscular deficits include a decrease in the neural activation of muscles (3, 28, 29, 36) and selective denervation of type IIa muscle fibers which are responsible for high force and rapid speed of contraction (9, 31, 57), together with increased adipose tissue infiltration and reduced quality of muscle fibers (34, 35, 52). Consequently, neuromuscular performance becomes impaired, particularly in the maximum rate of force development (RFD) and rate of neuromuscular activation (RActv) required for the generation of muscular power (1, 2, 11, 27, 38, 58). Although these aging changes generally affect the musculoskeletal system, the degree of decline may not be equivalent for all muscles. For example, compared to other muscles of the lower limbs, the gluteal musculature may be particularly susceptible to age-related alterations in muscle composition and performance (22, 24). Considering that hip abductor-adductor (AB-AD) muscles play a fundamental role in controlling body center of mass (CoM) motion in the medio-lateral (M-L) direction during standing, stepping, and walking (4, 39, 46, 50, 51), reduced AB-AD power generation may limit M-L balance and mobility functions that often require rapid, forceful, and controlled movements, such as protective steps in response to an unexpected balance perturbation.
In response to external postural perturbations, movements of the limbs such as stepping and reaching to grasp stable surfaces are normally pervasive balance stabilizing actions for protection against falls (30, 39, 41, 50). Moreover, successfully stepping sideways to recover balance is especially problematic for many older people with lateral instability (39), and may be attributable to a reduction in hip AB-AD muscle power generation, especially during the pre-step lateral weight transfer phase. This possibility may, at least in part, explain the increased use of multiple balance recovery steps and less stable M-L stepping strategies (e.g. crossover steps with inter-limb collisions) with older age and increased fall risk (5, 33, 39).
To counteract aging impairments in neuromuscular performance linked with balance and mobility deficits and falls, muscle strength training has been used as a fall prevention intervention strategy. Commonly, through a series of exercises and somewhat lengthy training sessions, this form of resistance exercise training is able to improve, to a certain extent, muscle strength, rate of force development, neuromuscular activation patterns and functional performance in older adults (2, 28, 32, 47, 56). However, strength training has generally been only modestly successful in improving balance and reducing fall risk (17–36% effective) (25, 54). It is conceivable that this limited effectiveness can be due to the lack of emphasis on rapid force production that is characteristic of power training and important for weight transfer during protective stepping (49). Moreover, muscle power has been shown to be better associated with functional independence and balance ability than muscle strength (17, 20, 37).
Recently, muscle power training has received increasing attention as a safe and effective alternative to strength training. While broadly similar to the existing strength training interventions in dosage and duration, power training improves muscular strength by emphasizing maximum speed of execution, but with greater improvements in muscular power, rate of neuromuscular activation and force development, and functional performance (7, 23, 32, 42, 48, 53). Furthermore, power training can increase muscle size and the number of type IIa myofibers, and enhance neuromuscular activation (18, 19). Intuitively, one might expect that power training-induced improvements in neuromuscular performance could be beneficial for balance stability involving rapid and forceful postural actions such as weight transfer and limb movements during M-L protective stepping. However, investigations of the effectiveness of training muscle power for improving balance ability and preventing falls have generally been sparse (8, 43, 44). While it has been suggested that a minimal-dose approach to resistance training may result in physiological and performance improvements (16), it is unknown whether M-L balance control through protective stepping can be enhanced through a low dosage of power training involving the hip AB-AD musculature.
To further address these issues, this study compared the effects of low-dose hip AB-AD strength and power training in community living older adults on: 1) the neuromuscular and mechanical performance during maximum voluntary isometric muscle contractions (IMVCs); and on 2) waist-pull induced lateral stepping at different initial limb pre-load conditions affecting weight transfer function and stepping. It was hypothesized that, compared with strength training, older individuals who performed hip power training would demonstrate greater improvements in AB-AD neuromuscular and mechanical performance, and a greater incidence of successful single lateral protective steps.
2. Methods
2.1. Participants
This study adopted a non-blinded randomized controlled trial design and based on the effect size (Cohen’s D) from similar work and unpublished data from our laboratory. To achieve a statistical power of at least 80%, each group would require approximately 8 participants. Hence, eighteen generally healthy older individuals were recruited from the Baltimore/Washington metropolitan area and randomly assigned to either a hip strength training group (ST, n=8 (3 males), 71.6 (1.7) yrs) or a hip power training group (PT, n=10 (5 males), 72.1 (1.3) yrs) (Table 1). Participants were recruited using an established recruitment registry, through advertisements, or by referral. After an initial phone screen, inclusion in the study was determined following a medical evaluation performed by a physician geriatrician. Exclusion criteria included the following: 1) cognitive impairment (Folstein Mini Mental Score Exam < 24); 2) sedative use; 3) non-ambulatory; 4) any clinically significant functional impairment related to musculoskeletal, neurological, cardiopulmonary, metabolic, or other general medical problem; 6) Centers for Epidemiological Studies Depression Survey score greater than 16; 7) BMI over 35; and 8) history of seizures or neurosurgery. All subjects provided written informed consent that was approved by the research ethics committee from the Institutional Review Board of University of Maryland, Baltimore and the Baltimore Veteran’s Administration Research and Development prior to participation.
Table 1.
Participant demographics
| Power Training (n=10) |
Strength Training (n=8) |
|
|---|---|---|
| Age (years) | 72.1 (1.3) | 71.6 (1.7) |
| Height (m) | 1.69 (0.02) | 1.66 (0.04) |
| Weight (kg) | 77.5 (4.9) | 74.6 (4.0) |
| BMI | 27.2 (1.5) | 27.1 (1.2) |
Data presented as Mean (SEM).
2.2. Muscle strength and power training protocol
The exercise intervention was preceded by a baseline testing session and followed by an identical post-training testing session. Strength training (ST) and power training (PT) groups performed a single hip AB and AD exercise while standing, using a pneumatic cable resistance Functional Trainer machine (KEISER, Fresno, CA, USA). Adapted from previously established protocols (10, 44), each protocol was performed for three sets of 10 repetitions, thrice-weekly, for eight weeks. Typically, each training session was very brief and lasted approximately 15 minutes. The resistance level was set at 75% of the subject’s one maximal repetition (1RM) that was assessed at the first training session and reassessed every other week to maintain the resistance load at a progressively challenging level. While the ST group performed each repetition at a slower speed of approximately 2s concentric and 3s eccentric phases of action, the PT group was instructed to perform every repetition at their maximum speed of execution.
2.3. Data collection
The isometric maximal voluntary contraction (IMVC) protocol consisted of three sets of five seconds each of bilateral hip AB-AD maximum isometric contractions performed as fast as possible on an isokinetic dynamometer (BIODEX, Shirley, NY, USA) for 5 seconds, with 90s of rest between sets. The hip was positioned at 30˚ of hip AB while standing in a customized stabilization frame (26).
For the stepping protocol, participants performed an initial “balance tolerance limit” (BTL) assessment (61), followed by induced lateral stepping trials at different initial stepping limb pre-load conditions. The lateral balance perturbations were applied via a motorized waist-pull system attached to a waist-belt previously described in detail (45). Participants wore a safety harness to protect them against falling (45).
The BTL assessment was performed to assess the lowest waist-pull intensity that resulted in more than one induced step on average (61). It consisted of forty randomly applied waist-pulls (5 trials × 2 sides × 4 pulling magnitudes), where participants were instructed to “react naturally and prevent themselves from falling”.
The induced stepping trials consisted of thirty randomly applied lateral waist-pulls to the side of the limb where the weight had been transferred initially (limb pre-load) (5 trials × 2 sides × 3 initial limb pre-load conditions). To minimize anticipation and postural adaptation, 12 additional catch trials (2 trials × 2 sides × 3 loading conditions) were delivered to the opposite side of the pre-loaded limb. Participants used online visual feedback from the force platforms to laterally transfer their weight to match 50%, 65%, or 80% of their body mass. Individuals were instructed to recover their balance using a single lateral step. The lateral balance perturbations occurred when the target body mass distribution was maintained for at least 50ms and were applied at one magnitude level above the previously determined BTL to increase the probability of inducing steps.
Kinetic data were recorded with two force platforms (AMTI, Watertown, MA, USA) at a collection frequency of 600Hz, and full body kinematic data were recorded via a motion capture system at 120Hz (VICON, Los Angeles, CA, USA). Reflective markers were placed bilaterally on the foot (first metatarsal, hallux, fifth metatarsal and calcaneus), ankle (lateral and medial malleoli), knee (lateral and medial femoral condyles), hip (greater trochanter of the femur, PSIS and ASIS), shoulders (acromions) and head (midpoint and center of parietal bones and most lateral point of temporal bones). Thus, a seven-segment model was created (bilateral foot, shank, thigh and HAT (head+arms+trunk)).
Surface electromyographic (EMG) (NORAXON, Scottsdale, AZ, USA) recordings of muscle activation patterns were collected during the hip AB-AD IMVC assessment and during the induced lateral stepping trials at a frequency of 1500Hz. The EMG electrodes were placed in accordance with established guidelines over the gluteus medius (Gmed), tensor fasciae latae (TFL), and adductor magnus (ADD) (12, 21) (Fig. 1). Kinetic, kinematic and EMG data collection onset was synchronized through an external trigger.
Fig. 1.
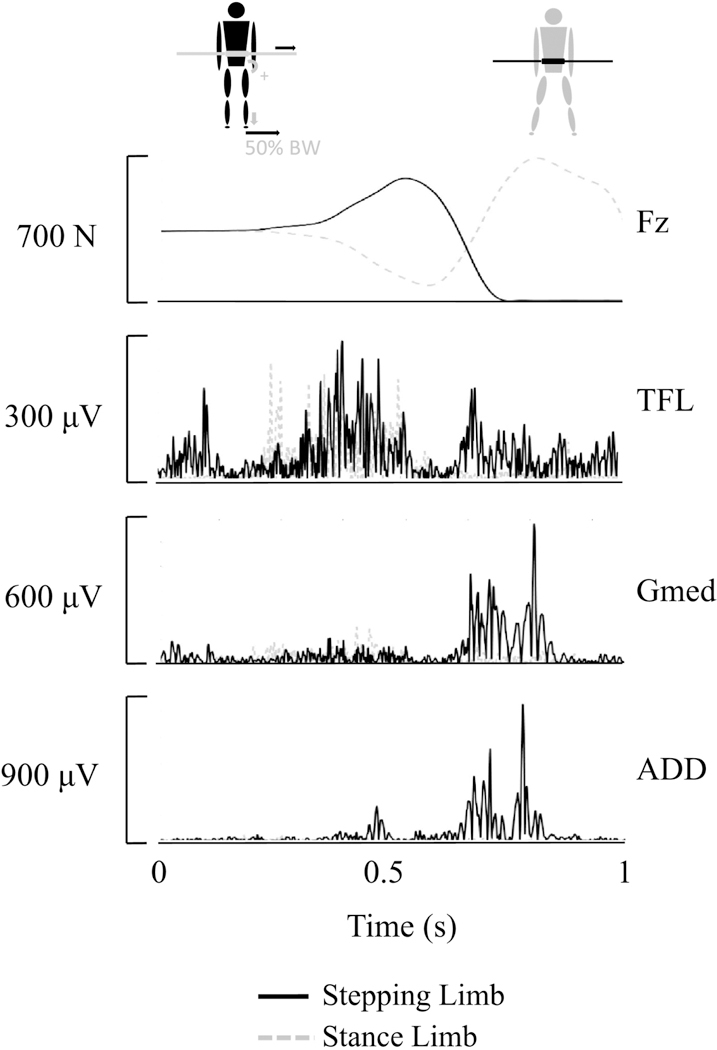
Vertical ground reaction force (Fz) and electromyography signals from the tensor fasciae latae (TFL), gluteus medius (Gmed) and adductor magnus (ADD), for the stance and the stepping limb during a representative induced lateral stepping trial.
2.4. Data Analysis
For the IMVC task, the rate of torque development (RTD) was calculated as the initial slope of the of torque-time curve by the following equation:
where, ∆T ‐ change in torque; ∆t ‐ change in time.
Peak torque was normalized to each individual’s height · weight.
All data analyses for the induced lateral stepping task targeted the weight transfer phase by focusing in the interval from the onset of lateral weight transfer to the instant of step lift-off. Weight transfer onset was defined as the instant where the vertical ground reaction force of the stepping foot was at least 3SD above the initial 100ms for each trial. Lift-off was defined as the instant where the stepping foot’s vertical ground reaction force equaled zero.
Kinetic and kinematic data were low-pass filtered at 16.5Hz to provide improved results in inverse dynamics computations (59). Rate of force development (RFD) was calculated as the slope of the stepping foot’s vertical ground reaction force from the onset of the weight transfer to the peak vertical force before lift-off, i.e. like that used for determining the RTD. Net hip AB-AD torque and power estimations were calculated using inverse dynamics derived from Newton-Euler equations of motion for determining net inter-segmental joint reaction forces and torques using a bottom-up approach where the ground reaction forces function as input of kinetic data to the first segment in the chain (foot) (13, 60). Torque and power estimates were normalized to each individual’s height · weight.
Body center of mass (CoM) momentum in the frontal plane was calculated by multiplying each individual’s mass with the CoM’s lateral velocity. Vertical CoM momentum was similarly calculated.
The raw EMG signals from each muscle were band-pass filtered (25–1000Hz), full-wave rectified, and low-pass filtered (6 Hz butterworth, 4th order) for smoothing purposes. Rate of neuromuscular activation (RActv) was defined as the initial slope of the EMG amplitude during phasic activation (11) and calculated similarly to RTD and RFD.
2.5. Statistical Analyses
The Shapiro-Wilk test was used to assess normality for the incidence of single lateral steps. A paired samples t-test was carried out for within-group comparisons of the incidence of lateral steps between pre and post training. Pre-post, within-group training differences for the IMVC and induced lateral stepping performance variables were determined with a linear mixed-effects model where the baseline and post-training tests (within groups) were fixed factors and the participants were random factors (SPSS v22, IBM, Armonk, NY). Significance level for all tests was set at p<0.05.
3. Results
3.1. Hip AB-AD Isometric Maximal Voluntary Contractions (IMVC)
Overall, the low dosage approach to hip AB-AD power training used in this study resulted in greater improvements than strength training during the IMVC task. Specifically, hip AB (14%) and AD (18%) peak torque increased significantly (p<0.05, Fig. 2 A). No significant effects due to strength training were found for the same performance variables (Fig. 2 A). Hip AB (39%) and AD (31%) IMVC rate of torque development also improved significantly for PT (p<0.05) but not ST (Fig. 2 B). Rate of neuromuscular activation of the TFL and ADD were significantly greater after PT (81% and 52% respectively, p<0.05, Fig. 2 C). The improvements with PT in Gmed RActv were marginally significant (37%, p=0.057, Fig. 2 C). ST did not result in any significant changes in RActv from pre-training (Fig. 2 C).
Fig. 2.
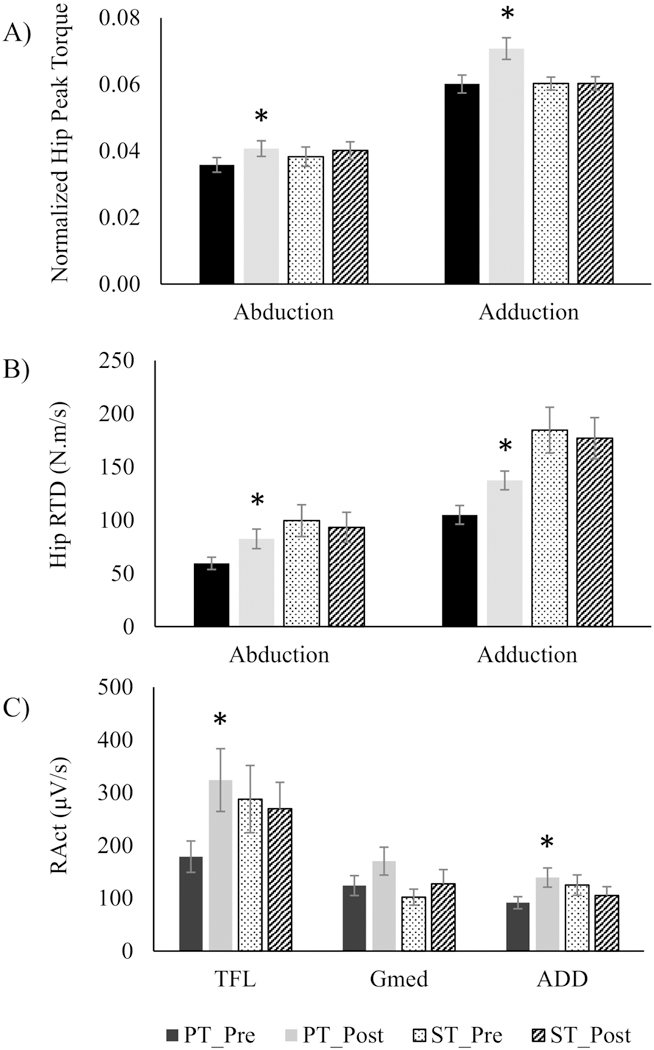
Normalized peak hip torque (A) and power (B), and neuromuscular rate of activation (RActv) of Tensor Fasciae Latae (TFL), Gluteus Medius (Gmed) and Adductor magnus (ADD), in power training (PT) and strength training (ST) during the isolated IMVC task. * significantly different from pre-training (p < 0.05)
3.2. Induced lateral stepping
Power training was also beneficial for recovering balance during the lateral induced stepping task. While the incidence of single lateral steps was not affected by ST in any pre-load condition (p>0.05), PT significantly increased the incidence of single lateral recovery steps by 43% in the 80% pre-load condition (p<0.05) and showed a trend for significance at the 65% BM (p=0.066) and 50% BM (p=0.093) conditions (Table 2).
Table 2.
Incidence of single lateral steps in hip power training (PT) and hip strength training (ST) during the lateral induced stepping task at different initial pre-loads.
| PT (n=10) | ST (n=8) | |||
|---|---|---|---|---|
| Lateral step incidence (%) | Lateral step incidence (%) | |||
| Pre | Post | Pre | Post | |
| 50% BW | 70.0 (11.3) | 81.0 (9.8) | 80.0 (6.5) | 81.3 (7.4) |
| 65% BW | 62.0 (9.0) | 76.0 (10.1) | 78.8 (9.5) | 68.8 (15.2) |
| 80% BW | 39.1 (10.6) | 56.0 (10.9)* | 55.0 (10.9) | 57.4 (12.7) |
Data presented as Mean (SEM).
significantly different from pre-training (p<0.05).
The lift-off time of the stepping foot during the 50% BM condition was reduced by 27ms (0.499 (0.010) s vs 0.472 (0.009) s, pre and post PT respectively, p<0.05), but was not altered by ST (p>0.05). Downward CoM momentum at step lift-off also decreased by 32% in the 80% pre-load condition (11.11 (1.40) kgm/s (pre) 7.49 (1.23) kgm/s (post), p<0.05) after PT, and increased after ST from 9.07 (1.78) kgm/s to 12.90 (1.49) kgm/s.
During the pre-step weight transfer phase, net hip AB torque (49%–61%, Fig. 3 A) and power (21%–54%, Fig. 3 B) were improved in the PT group in all pre-load conditions (p<0.05). In contrast, ST group had decreased AB torque in the 50% BM pre-load by 23% and by 18% in the 65% BM pre-load condition (p<0.05, Fig. 3 A). Similarly, AB power production was reduced after ST in the 50% BM pre-load condition (11%, p<0.05, Fig. 3 B).
Fig. 3.
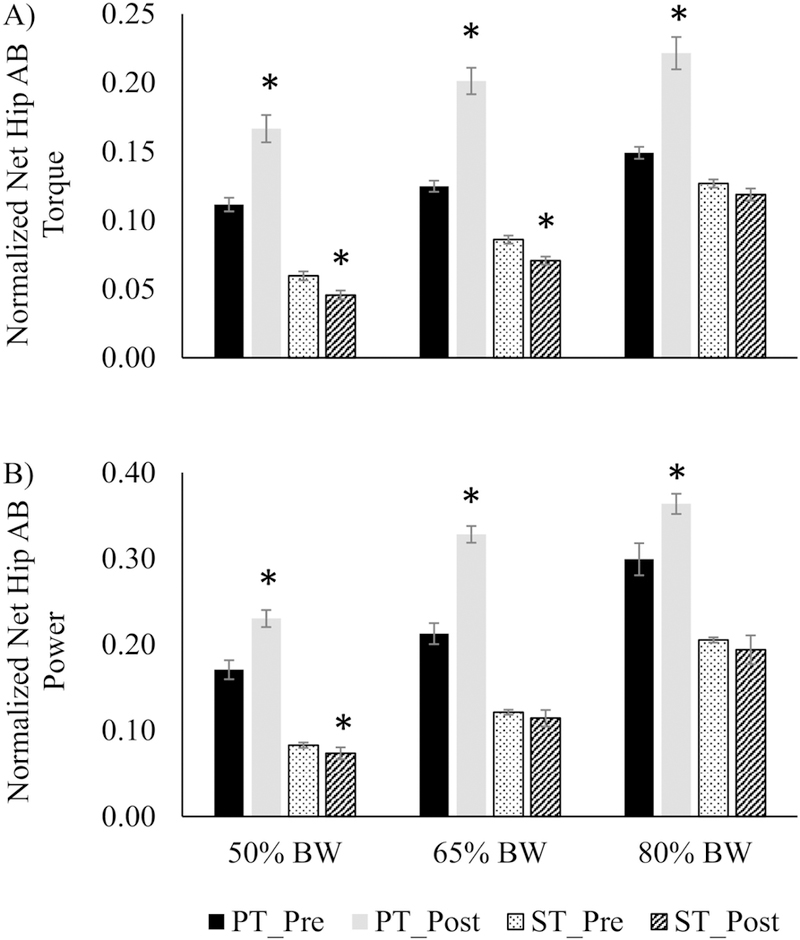
Normalized net hip abductor (AB) torque (A) and power (B), in power training (PT) and strength training (ST) during the lateral induced stepping task at different initial pre-loads. * significantly different from pre-training (p < 0.05)
EMG data further indicated that PT resulted in significant increases in RActv in all limb load conditions for TFL (22%–50%) and ADD (39%–62%) (p<0.05, Fig. 4 A, B, C). Gmed RActv improved after PT for 50% BM (33%) and 80% BM (43%) conditions (p<0.05, Fig. 4 A, C). In contrast, ST was associated with an observed decrease in ADD RActv at the 80% BM condition (p<0.05, Fig. 4 C).
Fig. 4.
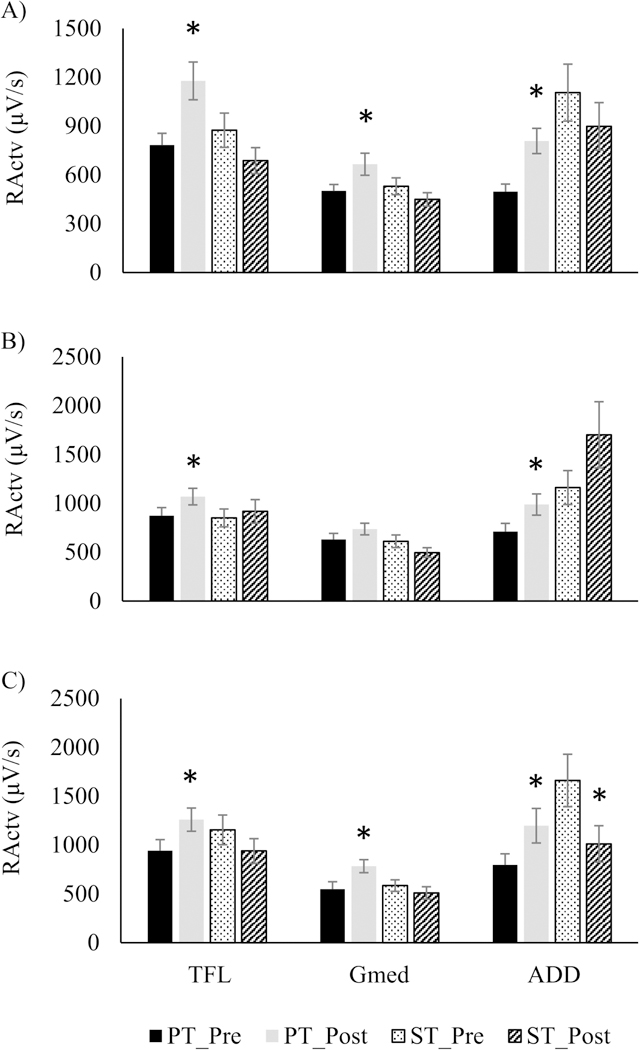
Neuromuscular rate of activation (RActv) of Tensor Fasciae Latae (TFL), Gluteus Medius (Gmed) and Adductor magnus (ADD), in power training (PT) and strength training (ST) during the lateral induced stepping task at 50% (A), 65% (B) and 80% (C) initial pre-loads. * significantly different from pre-training (p < 0.05)
4. Discussion
The present study demonstrated that low-dose hip abductor-adductor power training is a viable and effective alternative to strength training for eliciting improvements in hip AB-AD neuromuscular performance during isolated maximal contractions and induced lateral stepping.
4.1. Hip AB-AD Isometric Maximal Voluntary Contractions
Similar to previous reports (7, 18, 42, 48), this study’s low-dose application of hip AB-AD PT increased hip AB-AD maximal isometric performance through improvements in peak torque, and an even greater increase in the rate of torque development and neuromuscular activation. While resistance power training has been shown to increase muscle hypertrophy and the proportion of type IIa myofibers (18, 19), the observed gains were likely due to neural adaptation considering the relatively reduced duration and dosage of the low-dosage training approach. However, it is conceivable that some degree of muscular structural changes could also have contributed to the observed improvements in maximum muscle performance. Nonetheless, due to the emphasis on rapid speed of execution, PT increased the maximum RActv of the AB-AD musculature, a fundamental marker of neural drive (2, 14). This phenomenon results from adaptive changes in neuromuscular control in response to the high velocity requirements that were not elicited with slower velocity contractions performed during strength training (15). Increased neuromuscular rate of activation leads to improvements in maximum force and torque and is crucial for increasing the RFD/RTD and muscular power. Considering that impairments in muscle power generation have been associated with decreased mobility function and increased fall risk to a greater extent than muscle strength deficits (6, 17, 55), the present low-dose PT program appears to be an efficient and effective form of resistive exercise training for generally healthy community living older individuals. PT not only improved isolated maximum hip AB-AD muscle performance, but also enhanced neuromuscular control for improved balance recovery through protective stepping. The lack of improvements in maximum neuromuscular performance observed in the ST group is somewhat surprising, and is in contrast with the general findings for strength training programs (2, 32, 47, 56). Conceivably, it is possible that due to the neuromuscular mechanisms of force production inherent to slow execution speeds, the ST group would require a greater training dosage than what was achieved with the low-dose training approach to improve maximal isometric neuromuscular performance.
4.2. Induced lateral stepping
For lateral protective stepping, the PT group increased the incidence of single lateral steps by almost 50% at the most challenging 80% BM pre-load weight bearing condition, with similar trends observed for the 50% BM and 65% BM conditions. It is unlikely that these improvements in lateral stepping performance were attributable to changes in step onset timing during the weight transfer phase, as the time to step lift-off was just slightly shorter in only one of the pre-load conditions. However, the downward CoM momentum at step lift-off was reduced with PT at the 80% BM condition and a similar trend for 50% pre-load. This reflected improved balance control during weight transfer that resulted in a reduced “downward fall” at the instant of step onset. Furthermore, the PT group produced greater hip AB torque, hip AB power and AB-AD RActv. Thus, it appeared that the improved hip AB-AD performance capacity associated with PT enhanced lateral stepping ability through increased hip AB-AD neuromuscular and biomechanical control during the weight transfer phase. These neuromotor improvements allowed for greater success in executing sidesteps as evidenced by the increased incidence of single lateral steps, an important marker of M-L balance stability and fall risk following lateral postural perturbations (5, 33, 39, 40).
During the weight transfer phase of lateral stepping, the ST group often demonstrated opposite effects to those of the PT group. ST had increased CoM downward momentum, and decreased hip ADD RActv, hip AB torque and power. Considering these decreases in neuromotor performance and the lack of improvements in maximum AB-AD neuromuscular capacity, the unaltered incidence of lateral steps with ST is not surprising. We speculate that ST involving slower, sub-maximum speeds of contractile force may have reinforced neuromuscular mechanisms of force production that conflicted with the high speed-high force requirement for power generation during the weight transfer, and possibly contributed to the decreased neuromuscular performance in the lateral balance recovery task. This possibility may support the proposal that strength training, at least with the low-dose approach, is less effective than power training at improving balance function through stepping in older adults.
Among the limitations of this study was the relatively small sample size that may have affected some of the statistical analyses. Although the majority of the main outcome variables had sufficient statistical power, some of the secondary measures, such as CoM momentum and weight transfer timing, did not achieve significance for all of the pre-load conditions despite improving trends, and potentially might have benefited from a larger sample size. Lastly, the allocation of individuals in the training groups initially followed a randomization scheme. However, due to logistical limitations related to participant recruitment and the timeline for completion of the study, the randomization schedule was broken after participant 9 (of 18). This could have introduced selection bias in the training groups and potentially affected the outcomes. Nonetheless, the participants were volunteers that became aware of the study through either public advertisement or the recruitment registry. Hence, the recruitment of participants was done without the study team’s prior knowledge about those individuals (i.e. random extraction from the overall population), potentially minimizing the effects of selection bias.
In conclusion, a low-dose approach to muscle power training of the hip AB-AD musculature lasting about 15 minutes per training session was shown to be a viable and more effective alternative to strength training for enhancing maximal hip neuromuscular performance, and for improving the neuromotor control of pre-step weight transfer control during lateral balance recovery through protective stepping. Although, overall, ST did not result in significant improvements in isolated or balance-related neuromuscular or biomechanical performance, PT resulted in improved isolated maximal hip neuromotor performance needed to improve the recovery of lateral balance by using a single lateral step. Thus, PT increased the incidence of single lateral steps together with increased hip AB torque and power and AB-AD rate of neuromuscular activation. These findings have potentially important implications for the understanding of lateral balance function with aging. Furthermore, based on the neuromotor mechanisms of weight transfer and lateral stepping, these observations can help to guide more effective and efficient exercise training interventions for improving balance and reducing fall risk among older individuals.
Highlights.
-
-
Hip abductor-adductor power training was more effective than strength training;
-
-
Power training improved maximal hip abductor-adductor neuromuscular performance;
-
-
Power training enhanced weight transfer control and medio-lateral balance recovery.
Acknowledgements
Supported by NIH grants P30AG028747 and R01AG033607.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aagaard P, and Andersen JL. Correlation between contractile strength and myosin heavy chain isoform composition in human skeletal muscle. Med Sci Sports Exerc 30: 1217–1222, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, and Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93: 1318–1326, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Aagaard P, Suetta C, Caserotti P, Magnusson SP, and Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports 20: 49–64, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Addison O, Young P, Inacio M, Bair WN, Prettyman MG, Beamer BA, Ryan AS, and Rogers MW. Hip but not thigh intramuscular adipose tissue is associated with poor balance and increased temporal gait variability in older adults. Current aging science 7: 137–143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bair WN, Prettyman MG, Beamer BA, and Rogers MW. Kinematic and behavioral analyses of protective stepping strategies and risk for falls among community living older adults. Clin Biomech 36: 74–82, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, and Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci 58: 728–733, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Bottaro M, Machado SN, Nogueira W, Scales R, and Veloso J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol 99: 257–264, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gomez M, Rodriguez-Manas L, and Izquierdo M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 36: 773–785, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell MJ, McComas AJ, and Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caserotti P, Aagaard P, Larsen JB, and Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports 18: 773–782, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Clark DJ, Pojednic RM, Reid KF, Patten C, Pasha EP, Phillips EM, and Fielding RA. Longitudinal Decline of Neuromuscular Activation and Power in Healthy Older Adults. J Gerontol A Biol Sci Med Sci 2013. [DOI] [PMC free article] [PubMed]
- 12.De Luca CJ. The use of surface electromyography in biomechanics. Journal of Applied Biomechanics 13: 135–163, 1997. [Google Scholar]
- 13.Enoka RM. Neuromechanics of Human Movement Human Kinetics, 2002. [Google Scholar]
- 14.Farina D, Castronovo AM, Vujaklija I, Sturma A, Salminger S, Hofer C, and Aszmann O. Common Synaptic Input to Motor Neurons and Neural Drive to Targeted Reinnervated Muscles. The Journal of neuroscience the official journal of the Society for Neuroscience 37: 11285–11292, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, and Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc 50: 655–662, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fisher JP, Steele J, Gentil P, Giessing J, and Westcott WL. A minimal dose approach to resistance training for the older adult; the prophylactic for aging. Exp Gerontol 99: 80–86, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, and Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci 55: M192–199, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, Kraemer WJ, Newton RU, and Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. Journal of applied physiology 84: 1341–1349, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Hakkinen K, Kraemer WJ, Pakarinen A, Triplett-McBride T, McBride JM, Hakkinen A, Alen M, McGuigan MR, Bronks R, and Newton RU. Effects of heavy resistance/power training on maximal strength, muscle morphology, and hormonal response patterns in 60–75-year-old men and women. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee 27: 213–231, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Han L, and Yang F. Strength or power, which is more important to prevent slip-related falls? Human movement science 44: 192–200, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Hermens HJ, Freriks B, Disselhorst-Klug C, and Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, and Rogers MW. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil 89: 1708–1713, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst C, Weston KL, and Weston M. The effect of 12 weeks of combined upper- and lower-body high-intensity interval training on muscular and cardiorespiratory fitness in older adults. Aging Clin Exp Res 2018. [DOI] [PMC free article] [PubMed]
- 24.Inacio M, Ryan AS, Bair WN, Prettyman M, Beamer BA, and Rogers MW. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC geriatrics 14: 37, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishigaki EY, Ramos LG, Carvalho ES, and Lunardi AC. Effectiveness of muscle strengthening and description of protocols for preventing falls in the elderly: a systematic review. Brazilian journal of physical therapy 18: 111–118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson ME, Mille ML, Martinez KM, Crombie G, and Rogers MW. Age-related changes in hip abductor and adductor joint torques. Arch Phys Med Rehabil 85: 593–597, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kallman DA, Plato CC, and Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol 45: M82–88, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Kanda K, Hashizume K, Miwa T, and Miwa Y. Overloading a muscle does not alter the rate of motoneuronal loss in aged rats. Neurobiol Aging 17: 613–617, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Kido A, Tanaka N, and Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82: 238–248, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Lattimer LJ, Lanovaz JL, Farthing JP, Madill S, Kim SY, Robinovitch S, and Arnold CM. Biomechanical and physiological age differences in a simulated forward fall on outstretched hands in women. Clin Biomech (Bristol, Avon) 52: 102–108, 2018. [DOI] [PubMed] [Google Scholar]
- 31.Lexell J, and Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Lopes PB, Pereira G, Lodovico A, Bento PC, and Rodacki AL. Strength and Power Training Effects on Lower Limb Force, Functional Capacity, and Static and Dynamic Balance in Older Female Adults. Rejuvenation research 2016. [DOI] [PubMed]
- 33.Maki BE, Edmondstone MA, and McIlroy WE. Age-related differences in laterally directed compensatory stepping behavior. J Gerontol A Biol Sci Med Sci 55: M270–277, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, and Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012: 629637, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus RL, Addison O, Kidde JP, Dibble LE, and Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 14: 362–366, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeil CJ, Doherty TJ, Stashuk DW, and Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. [DOI] [PubMed] [Google Scholar]
- 37.McNeil CJ, Vandervoort AA, and Rice CL. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol 102: 1962–1968, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Metter EJ, Conwit R, Tobin J, and Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 52: B267–276, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Mille ML, Johnson-Hilliard M, Martinez KM, Zhang Y, Edwards BJ, and Rogers MW. One step, two steps, three steps more Directional vulnerability to falls in community-dwelling older people. J Gerontol A Biol Sci Med Sci 68: 1540–1548, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mille ML, Johnson ME, Martinez KM, and Rogers MW. Age-dependent differences in lateral balance recovery through protective stepping. Clin Biomech 20: 607–616, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Moon Y, and Sosnoff JJ. Safe Landing Strategies During a Fall: Systematic Review and Meta-Analysis. Arch Phys Med Rehabil 98: 783–794, 2017. [DOI] [PubMed] [Google Scholar]
- 42.Nogueira W, Gentil P, Mello SN, Oliveira RJ, Bezerra AJ, and Bottaro M. Effects of power training on muscle thickness of older men. International journal of sports medicine 30: 200–204, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, and Fiatarone-Singh MA. Power training improves balance in healthy older adults. J Gerontol A Biol Sci Med Sci 61: 78–85, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Pamukoff DN, Haakonssen EC, Zaccaria JA, Madigan ML, Miller ME, and Marsh AP. The effects of strength and power training on single-step balance recovery in older adults: a preliminary study. Clinical interventions in aging 9: 697–704, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pidcoe PE, and Rogers MW. A closed-loop stepper motor waist-pull system for inducing protective stepping in humans. Journal of biomechanics 31: 377–381, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Porto JM, Freire Junior RC, Bocarde L, Fernandes JA, Marques NR, Rodrigues NC, and de Abreu DCC. Contribution of hip abductor-adductor muscles on static and dynamic balance of community-dwelling older adults. Aging Clin Exp Res 2018. [DOI] [PubMed]
- 47.Ramirez-Campillo R, Castillo A, de la Fuente CI, Campos-Jara C, Andrade DC, Alvarez C, Martinez C, Castro-Sepulveda M, Pereira A, Marques MC, and Izquierdo M. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp Gerontol 58: 51–57, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Reid KF, Callahan DM, Carabello RJ, Phillips EM, Frontera WR, and Fielding RA. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res 20: 337–343, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid KF, and Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, Sims-Gould J, and Loughin M. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet 381: 47–54, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers MW, and Mille ML. Lateral stability and falls in older people. Exerc Sport Sci Rev 31: 182–187, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Ryan AS, and Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord 23: 126–132, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Sayers SP, and Gibson K. A comparison of high-speed power training and traditional slow-speed resistance training in older men and women. Journal of strength and conditioning research National Strength & Conditioning Association 24: 3369–3380, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, and Close JC. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 56: 2234–2243, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Skelton DA, Kennedy J, and Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing 31: 119–125, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, and Magnusson SP. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol 97: 1954–1961, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Tam SL, and Gordon T. Mechanisms controlling axonal sprouting at the neuromuscular junction. J Neurocytol 32: 961–974, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Thompson BJ, Conchola EC, Palmer TB, and Stock MS. Effects of aging on maximal and rapid velocity capacities of the leg extensors. Exp Gerontol 58: 128–131, 2014. [DOI] [PubMed] [Google Scholar]
- 59.van den Bogert AJ, and de Koning JJ. ON OPTIMAL FILTERING FOR INVERSE DYNAMICS ANALYSIS. Proceedings of the IXth Biennial Conference of the Canadian Society for Biomechanics 214–215, 1996.
- 60.Winter DA. Biomechanics and Motor Control of Human Movements Waterloo, Canada: University of Waterloo Press, 1992. [Google Scholar]
- 61.Yungher DA, Morgia J, Bair WN, Inacio M, Beamer BA, Prettyman MG, and Rogers MW. Short-term changes in protective stepping for lateral balance recovery in older adults. Clin Biomech 27: 151–157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


