Abstract
Gestational diabetes mellitus (GDM) has emerged as a global public health problem, both globally and in India. Despite government of India (GoI) prior mandate to screen all Indian pregnant women for GDM, its real operationalization at primary health-care level is suboptimal. Adding new operational component, GoI revised its existing recommendations and released new technical operational guidelines on GDM diagnosis and management in February 2018. The revised guideline highlights integration of two vertical programs, ensuring effective GDM service delivery to all antenatal women at every pause point of existing service delivery platform within public health system. However, its real success depends on knowledge and commitment level of health-care providers. Despite maternal and child health holding high public health relevance since long, community-level delivery of services still persists to be at high risk of fragmentation and inefficiency.
Keywords: 2018, gestational diabetes mellitus, maternal and child health, primary care physicians, technical and operational guidelines
Gestational Diabetes Mellitus – indian Context
Gestational diabetes mellitus (GDM), defined as diabetes diagnosed in second and third trimesters of pregnancy,[1] has emerged as a global public health problem.[2] In India alone, GDM complicates nearly 4 million pregnancies annually, representing large subset of population at high risk for adverse perinatal morbidity and mortality if left inappropriately managed.[2] Beyond perinatal implications, GDM marks beginning of a vicious cycle in which diabetes begets more diabetes,[3,4] leaving a legacy for both affected mother and her offspring to face impending long-term consequences. As substantiated by “fetal origin of adult disease” hypothesis,[5] perpetuation of this ongoing cycle needs check to avoid occurrence of unfavorable consequences in future generations. Considering wide range of GDM prevalence in the country, its early identification assumed national significance.[6] Despite government of India (GoI) prior mandate to screen all Indian pregnant women for GDM as a part of routine antenatal package according to country's 2014 national guidelines,[6] its real operationalization at primary health-care level is still unclear. A recent community-based study in Tamil Nadu revealed GDM prevalence at three settings: 17.8% in urban, 13.8% in semi-urban, and 9.9% in rural areas.[7] However, in public health facilities of Bangalore, the study reported GDM prevalence <1%.[8] This considerable underestimation of GDM prevalence in government facilities was partly explained by district level health survey (DLHS) findings. As per DLHS-4 (2012–2013), only 59% of government health services in Bangalore were utilized for providing complete antenatal check-up.[9] Among them, only 70% provided laboratory facilities to screen GDM. Less than half of government doctors (44%) employed oral glucose tolerance test (OGTT) to diagnose GDM with mere 74% prescribing recommended 75 g glucose. Few utilized 50 g (18%) and 100 g (8%) glucose for performing OGTT. Most doctors were utilizing other screening tests like random blood glucose (46%), fasting plasma glucose (18%), and postprandial blood glucose (12%) for diagnosing GDM, outside recommended national guidelines.[8]
India – Recent Guidelines
Though country has shown improved antenatal coverage in past two decades, universal GDM screening is yet to be attained. Old national guideline came into force in 2014 when GoI formulated local guidelines specific to Indian population pertaining to GDM diagnosis and its management. That time, it met the need of hour especially when there existed no uniform consensus regarding standard protocols on GDM diagnosis despite availability of various international guidelines. For the sake of simplicity, economical, and feasibility, GoI endorses Diabetes in Pregnancy Study Group India (DIPSI) criteria and employed single-step procedure in guidelines for GDM diagnosis. In accordance to DIPSI criteria, 2-h 75-g OGTT was employed to all pregnant women irrespective of their last meal timings.[6] Although the criteria for GDM screening and diagnosis were established, uncertainty still existed on its execution methods.
To meet current logistics limitations and technical advances of country, domain experts from GoI revised existing recommendations and released new technical operational guidelines on GDM diagnosis and its management in February 2018.[10] Approach to subject all pregnant women for GDM testing twice during antenatal period irrespective of their risk profile remains central recommendation of this guideline as well.[10] However, recent guideline also added a detailed account on operational aspects regarding GDM-related service delivery at field level. It also highlighted the importance of integrated delivery with already existing two national programs – Reproductive and Child Health (RCH) Programme and National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke (NPCDCS). Such integration will help in effective GDM service delivery to all antenatal women as a part of essential antenatal packaged services at every pause point of existing service delivery platform within public health system.[10] In general, these new recommendations are based on Indian-specific preventive strategies that focus on early GDM identification, averting any chance of related complications that would otherwise appear if left undiagnosed. Effective service delivery was ensured by utilizing already existing platform provided by above two programs, avoiding overlap and duplication of services, thus making it more cost-effective. Losing no opportunity to miss the diagnosis, this strategy attempts to provide wide cover to major section of Indian population by protecting against vertical transmission of disease, preventing intergenerational development of chronic diseases. In the wake of recent changes in operational aspects of GDM diagnosis and management in India, we would like to bring out major highlights of these new technical guidelines released in February 2018.
It emphasizes nation-wide rolling out of GDM services in phased manner to ensure GDM service delivery in all districts by the end of next 5 years. Initial target is on high-priority districts, later extending to remaining districts of the country. For effective implementation, guidelines envisage integration of GDM services with essential continuum of care package. This includes service delivery at following pause points in continuum of care: antepartum, intrapartum, immediate postpartum, and early neonatal phase. They cover activities pertaining to demand generation, early diagnosis of GDM and its management, and establishment of maternal health and Non-communicable disease (NCD) program linkages. Table 1 summarizes the role of involved health personnel at different levels of health facility.
Table 1.
Role of health personnel involved at different levels of health facility
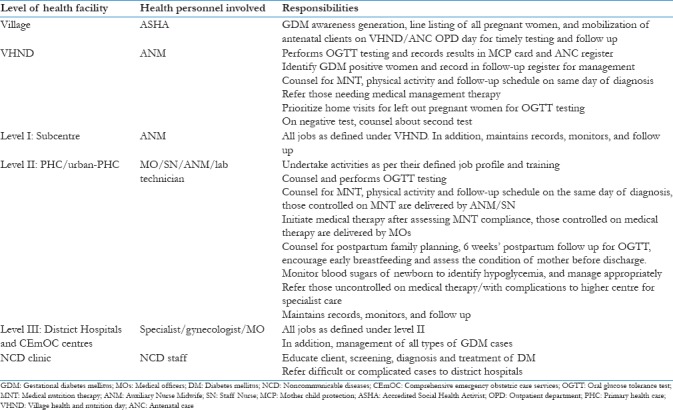
Gestational Diabetes Mellitus Care Provision under Revised Guidelines
GDM-specific activities carried out at these pause points are described as follows.
Antenatal care
It encompasses universal screening for GDM among all pregnant women, shortly at first antenatal contact. It includes line listing of all expecting women in the village, generating awareness to create demand for GDM testing at community level.
Definition and Screening
GDM is operationally defined as impaired glucose tolerance with onset or first recognition during pregnancy. Its diagnosis is based on single-step procedure. In accordance to World Health Organization recommendations, the guideline endorses 2-h 75-g OGTT, irrespective of last meal timings with a cutoff value of ≥140 mg/dL using a plasma-standardized glucometer. Peripheral health worker (ANM/ASHA) performs this test for each pregnant woman and records her results in mother–child protection (MCP) card. A red sticker is pasted onto MCP cards for all identified GDM-positive women based on OGTT results. Newly identified GDM-positive women are registered for subsequent management and follow-up. If woman tested positive (2-h 75-g OGTT ≥140 mg/dL), she is managed as per GDM protocols. However, if tested negative, OGTT is repeated at 24–28 weeks ensuring a minimum gap of at least 4 weeks from the first test. It is prudent to ensure second test as many pregnant women develop glucose intolerance during this period. If woman presents beyond 28 weeks, only one test is performed at first point of contact irrespective of gestation. GDM testing is performed at Pradhan Mantri Surakshit Matritva Abhiyan sites. For those who drop out, field health workers make special house visits to perform OGTT among such women.
Clinical Management
GDM is managed through medical nutrition therapy (MNT) along with physical activity, followed by subsequent 2-h postprandial blood sugar (PPBS) testing at 2 weeks. Two-hour PPBS level is maintained below <120 mg/dL. If 2-h PPBS remains ≥120 mg/dL, medical therapy (insulin/metformin) is added to MNT as per the guidelines. Metformin (oral antidiabetic drug) or insulin therapy is the accepted medical management for GDM. Insulin therapy can be started anytime during pregnancy whereas metformin can be initiated only at/after 20 weeks. However, if blood sugars are uncontrolled (2-h PPBS ≥120 mg/dL) with maximum dose of metformin (2 g/day), insulin therapy is added. Dose of insulin/metformin is titrated as per blood sugar level and follow-up schedule. Monitoring fasting blood sugar (FBS) and 2-h PPBS is done every third day or more frequently for insulin and bi-weekly for metformin dose adjustment to maintain normal blood sugar levels. GDM positivity is recognized as high-risk pregnancy. During each antenatal visit, GDM woman is monitored for abnormal fetal growth (macrosomia/growth restriction), polyhydramnios, hypertension in pregnancy, proteinuria, and other obstetric complications. Fetal heart sound is heard at each antenatal visit and ultrasonography (USG) is performed thrice during entire antenatal period: one at 18–20 weeks for fetal anatomical survey, followed by two growth scans at 28–30 and 34–36 weeks, respectively. USG also includes fetal biometry and amniotic fluid estimation. Each USG is separated by a minimum gap of at least 3 weeks. Follow-up visits are ensured for regular blood sugar monitoring as per specified “high-risk pregnancy” protocol. It includes additional four ANC visits, along with four routine visits (at least every month) until delivery and during sixth week postpartum. However, if blood sugars are uncontrolled or in view of any danger signs, GDM woman is referred to higher center/comprehensive emergency obstetric care services (CEmOC) centers where obstetrician is available.
Intrapartum Care
Woman on medical management requires strict blood sugar monitoring during labor using glucometer. Therefore, universal institutional delivery is promoted and vaginal delivery is preferred. However, caesarian section is performed only under obstetric conditions or fetal macrosomia (estimated fetal weight >4 kg) to avoid shoulder dystocia. As GDM pregnancy is associated with delayed fetal lung maturity risk, routine delivery prior to 39 weeks is not recommended. Early delivery prior to 34 weeks of gestation is planned with administration of prophylactic corticosteroid therapy only if uncontrolled blood sugar (PPBS ≥120 mg/dL) or any other obstetric indication exists. Those on medical therapy with uncontrolled blood sugars or on daily insulin requirement >20 U are referred to higher facility/CEmOC for delivery planning.
Immediate Newborn Care
Essential maternal and neonatal care is important for all GDM women. All babies born to GDM mothers are evaluated for immediate hypoglycemia (<45 mg/dL) within first hour of birth and at 4 hours interval using glucometer till four stable readings glucose values are achieved (≥45 mg/dL). In addition, newborns are monitored for respiratory distress, convulsions, and hyperbilirubinemia. Exclusive breast-feeding is promoted preferably by direct breast-feeding. In case, blood sugar falls <20 mg/dL, infant is referred to higher center with 10% dextrose IV infusion drip (100 mL/kg/day) where pediatrician is available.
Postpartum Care
All GDM mothers are clinically assessed in detail and are counseled about lifestyle modifications, warning signs, weight monitoring, exercise, and postpartum family planning prior to discharge. Guideline endorses GDM to be a part of NCD program. GDM mother is also counseled about postpartum follow-up testing at 6 weeks by 75-g OGTT (fasting and 2-h PPBS) to evaluate her glycemic status (normal <140 mg/dL). These are later linked to NCD clinics for regular annual follow-up screening for Type II DM postdelivery at various existing platforms, namely, NCD clinic, postpartum care clinic, pediatric setups as per programmatic protocols.
Preconception care and counseling is offered to all GDM mothers to ensure desired FBS (<100 mg/dL) and 2-h PPBS levels (<140 mg/dL) before conceiving again. She is also advised to consult an obstetrician as soon as she misses her period.
Guideline envisages provision of plasma calibrated glucometer with strips, 40 IU insulin syringe/insulin pen, calibration fluids, lancets, cotton swabs, disposable 300 mL glasses, spoons, 500 mL beakers with markings, and drugs like metformin, insulin at all antenatal clinics and labor rooms located at medical colleges, district hospitals, and other CEmOC centers with facility for sample collection and results interpretation. At remaining health-care facilities up to PHC level, an in-house arrangement of glucometer and 75 g glucose pouches are mandated for conducting test and providing report immediately. This is done so that necessary advice can be given on the same day of testing.
Integration of guidelines with comprehensive primary care services
Diabetes is a primary care disease. Majority of GDM subjects can be managed within the community. However, real success of these recent guidelines depends on knowledge and commitment level of health-care providers. Role of primary care physicians, along with primary health-care teams, for achieving universal GDM identification in community, ensuring adequate management, and prompt referral to higher centers is of paramount importance. In addition, their responsibility to ensure compliance and health education interventions will help improving outcomes. Their core committed involvement in GDM prevention, control, and management is utmost essential to make this guideline a grand success. The abovementioned new recommendations will be helpful in standardizing GDM care at community settings, thereby minimizing adverse impacts of unmanaged GDM. These tasks have to be inherently placed within the existing comprehensive skill set and clinical roles of primary health-care teams.
However, in India, the traditional public approach has been to offer program-based solutions in a piecemeal manner. The fact that two vertical national programs – RCH and NPCDCS – are involved is a reflection of design challenge. Though maternal and child health holds high public health priority, community-level services delivery are at high risk of fragmentation and inefficiency. Strengthening selective clinical care for one component of population without synergy with other existing similar programs will only lead to resource wastage. Primary care with best outcomes is always delivered when services are comprehensive, continuity of care is present, and care is person-centered.
Implementation of such guidelines will remain challenging at ground level till delivery of comprehensive primary care services is redesigned and oriented to address population (nonselective) needs approach rather than outdated delivery-focused model of vertical programs. Clinical guidelines will be useful for training of primary care doctors and teams; however, as program component, it is likely to remain challenging.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Diabetes Association. (2) classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl 1):S8–16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–85. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization; 2011. [Last accessed on 2018 Jul 10]. World Health Organization. Global Status Report on Non-Communicable Diseases 2010: Description of the Global Burden of NCDs, their Risk Factors and Determinants. Available from: http://www.apps.who.int/iris/bitstream/10665/44579/1/9789240686458_eng.pdf . [Google Scholar]
- 4.Matyka KA. Type 2 diabetes in childhood: Epidemiological and clinical aspects. Br Med Bull. 2008;86:59–75. doi: 10.1093/bmb/ldn018. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Government of India. Maternal and Health Division, National Guidelines for Diagnosis and Management of Gestational Diabetes Mellitus. New Delhi, India: Ministry of Health & Family Welfare, New Concept Information Systems; 2014. [Google Scholar]
- 7.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) – A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 8.Babu GR, Tejaswi B, Kalavathi M, Vatsala GM, Murthy GV, Kinra S, et al. Assessment of screening practices for gestational hyperglycaemia in public health facilities: A descriptive study in Bangalore, India. J Public Health Res. 2015;4:448. doi: 10.4081/jphr.2015.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Government of India. District Level Household and Facility Survey-4. District Fact Sheet. Bangalore, India: Ministry of Health & Family Welfare, International Institute for Population Sciences; 2012. [Google Scholar]
- 10.Government of India. Maternal and Health Division, Diagnosis and Management of Gestational Diabetes Mellitus: Technical and Operational Guidelines. New Delhi, India: Ministry of Health & Family Welfare, New Concept Information Systems; 2018. [Google Scholar]


