Abstract
Background
Oxytocin (OT) and arginine vasopressin (AVP) exert sexually dimorphic effects on cognition and emotion processing. Abnormalities in these hormones are observed in schizophrenia and may contribute to multiple established sex differences associated with the disorder. Here we examined sex-dependent hormone associations with resting brain activity and their clinical associations in schizophrenia patients.
Methods
OT and AVP serum concentrations were assayed in 35 individuals with schizophrenia (23 men) and 60 controls (24 men) from the Chicago BSNIP study site. Regional cerebral function was assessed with resting state fMRI by measuring the amplitude of low-frequency fluctuations (ALFF) which are believed to reflect intrinsic spontaneous neuronal activity.
Results
In female patients, lower OT levels were associated with lower ALFF in frontal and cerebellar cortices (p’s<0.05) and in female controls AVP levels were inversely associated with ALFF in the frontal cortex (p=0.01). In male patients, lower OT levels were associated with lower ALFF in the posterior cingulate and lower AVP levels were associated with lower ALFF in frontal cortex (p’s<0.05). In male controls, lower OT levels were associated with lower ALFF in frontal cortex and higher ALFF in the thalamus (p’s<0.05). There were some inverse ALFF-behavior associations in patients.
Conclusions
Alterations in peripheral hormone levels are associated with resting brain physiology in a sex-dependent manner in schizophrenia. These effects may contribute to sex differences in psychiatric symptom severity and course of illness in schizophrenia.
Keywords: sex differences, resting state, schizophrenia, oxytocin, vasopressin, functional MRI
1. Introduction
Sex differences in schizophrenia have been reported in brain structure and function, cognition, emotion processing, and course of illness. Normal brain sexual dimorphisms are disrupted in schizophrenia (Goldstein, 1996; Goldstein et al., 2002; Gur et al., 2004). The course of illness is also less severe in women than men; females on average have a later age of onset, briefer and less frequent acute episodes of illness, less severe negative symptoms, better premorbid functioning, and a better treatment response to antipsychotic medication versus males (Aleman et al., 2003; Goldstein and Walder, 2006; Grigoriadis and Seeman, 2002; Hafner, 2003). Hormonal abnormalities are also known to differ in male and female patients, and may contribute to sex differences in brain and behavioral functions in individuals with schizophrenia.
Two sexually dimorphic neurohormones—oxytocin (OT) and arginine vasopressin (AVP)—may be important contributors to clinically-observed sex differences in schizophrenia. First, there are alterations in OT and AVP systems in schizophrenia (Rubin et al., 2014). Second, these hormones are known to be differentially important contributors to resting brain function in healthy individuals, and these effects are seen in networks important for emotion and cognition (Rubin et al., 2017) and which are impacted in schizophrenia (Lui et al., 2015). For example, in healthy individuals higher AVP levels in men and higher OT levels in women have been associated with reductions in functional network connectivity of the orbital frontal cortex, which is important in emotion regulation (Rubin et al., 2017).
Little is known about how the OT and AVP systems might influence brain physiology in women and men with schizophrenia. Overlap in the brain regions that these hormones modulate in healthy individuals and those known to be abnormal in individuals with schizophrenia suggest that OT and AVP may contribute to alterations in brain function that in turn may contribute to behavioral alterations associated with the illness. Further, these associations may differ in male and female patients; hormone-behavior associations for OT and AVP have been shown to be sex dependent in previous studies (Rubin et al., 2014; Rubin et al., 2013; Rubin et al., 2011; Rubin et al., 2010; Rubin et al., 2015).
In the present study, we examined whether hormone-brain physiology associations are altered in men and women with schizophrenia compared to healthy male and female controls. Regional cerebral function was assessed with resting state functional magnetic resonance imaging (fMRI) by measuring the amplitude of low-frequency fluctuations (ALFF) which are believed to reflect spontaneous neuronal activity during rest. We predicted that basal levels of OT and AVP would be differentially associated with ALFF in females and males with schizophrenia compared to same sex controls. Given findings from pharmacological fMRI studies in healthy individuals (Tully et al., 2018; Wang et al., 2017), particularly those examining either OT administration on functional connectivity (Ebner et al., 2016; Eckstein et al., 2017; Sripada et al., 2013) and studies examining associations between basal OT levels to task-based fMRI (Lancaster et al., 2015), we expected that the strongest correlates for basal OT levels would be frontal and limbic regions as well as cingulate cortex. Fewer studies have focused on the impact of AVP administration on task-based fMRI (Chen et al., 2016; Lee et al., 2013; Rilling et al., 2014). To our knowledge, no studies to date have examined AVP administration on resting state fMRI and only two studies to date examined basal AVP levels in relation to resting state fMRI (Rubin et al., 2017; Shou et al., 2017). Based on these studies, we expected that the strongest correlates of AVP levels would be frontal and limbic regions as well as the insula and supramarginal gyrus. Secondarily, we examined associations of hormone brain physiology findings with symptom severity, cognition, and emotion processing.
2. Methods
2.1. Participants
Participants included 35 individuals with DSM-IV diagnoses of schizophrenia (12 women, 23 men) and 60 healthy individuals (36 women, 24 men) from the Chicago site of the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium at which plasma for endocrine assays was obtained. Recruitment, diagnostic strategies and patient characteristics have been described previously (Tamminga et al., 2013)(Table 1). All participants provided written informed consent and the study was approved by the University of Illinois at Chicago Institutional Review Board.
Table 1.
Demographics and Clinical Characteristics by Group and Sex.
| Women | Men | |||
|---|---|---|---|---|
|
| ||||
| Patients (n=12) | Healthy Controls (n=33) | Patients (n=23) | Healthy Controls (n=24) | |
| Demographics | ||||
| Age (years), M (SD) B | 40.8 (14.2) | 35.2 (12.5) | 37.3 (10.9) | 30.0 (10.7) |
| Education (years), M (SD)A, B | 13.0 (2.5) | 15.3 (2.7) | 13.3 (2.7) | 15.0 (2.4) |
| WRAT Reading, M (SD) | 99.2 (19.0) | 103.2 (14.9) | 94.0 (15.2) | 100.8 (13.8) |
| Race, n (%) | ||||
| Caucasian | 4 (33) | 20 (61) | 12 (50) | 13 (56) |
| African-American | 7 (59) | 9 (27) | 8 (33) | 8 (35) |
| Other | 1 (8) | 4 (12) | 4 (17) | 2 (9) |
| Right Handed, n (%) | 10 (83) | 30 (91) | 22 (92) | 21 (91) |
| Blood draw ≤ noon, n (%) | 11 (96) | 33 (100) | 23 (96) | 22 (96) |
| Hormone levels, M (SD) | ||||
| Mean log oxytocin (pg/ml) | 6.2 (0.8) | 6.1 (0.9) | 5.7 (0.6) | 6.1 (0.7) |
| Mean log vasopressin (pg/ml) A | 3.9 (0.5) | 4.5 (0.5) | 4.2 (0.6) | 4.3 (0.7) |
| Emotion and cognitive outcomes, M (SD) | ||||
| Emotion processing A | 75.9 (8.0) | 85.7 (5.6) | 74.5 (15.1) | 81.1 (9.3) |
| Verbal learning B | 47.0 (9.4) | 49.8 (8.5) | 36.6 (10.6) | 43.2 (9.1) |
| Phonemic fluency | 29.5 (12.3) | 31.3 (8.1) | 25.2 (9.8) | 29.3 (8.6) |
| Semantic fluency B | 21.1 (6.3) | 24.3 (5.8) | 18.5 (5.1) | 22.6 (7.2) |
| Processing speed A,B | 53.6 (13.6) | 61.0 (10.0) | 45.1 (13.2) | 56.7 (14.9) |
| Symptomatology, M (SD) | ||||
| SFSA | 126.3 (22.8) | 162.7 (16.8) | 126.6 (22.1) | 168.6 (15.0) |
| PANSS | ||||
| Positive subscale | 19.4 (5.7) | 18.7 (5.8) | ||
| Negative subscale | 18.4 (6.4) | 20.7 (7.3) | ||
| YMRS | 8.2 (5.4) | 6.8 (7.2) | ||
| MADRS | 8.7 (8.4) | 8.7 (6.9) | ||
| Schizo-Bipolar Scale | 7.2 (1.6) | 8.0 (1.3) | ||
| Age, Onset of psychotic illness Hospitalizations | ||||
| Medication ClassC, n (%) | ||||
| Antipsychotic | 9 (75) | 23 (96) | ||
| First generation | 2 (17) | 2 (8) | ||
| Second generation | 7 (58) | 21 (87) | ||
| Mood stabilizer | - | 7 (29) | ||
| Antidepressant | 4 (33) | 10 (42) | ||
| Sedative/anxiolytic/hypnotic | 5 (42) | 7 (29) | ||
| Stimulant | 2 (17) | 2 (8) | ||
| Anticholinergic | - | 2 (8) | ||
| Chlorpromazine equivalent, Median (IQR) | 395.8 (285) | 507.8 (649) | ||
Note.
group difference among women at p<0.05;
group difference among males at p<0.05. WRAT, Wide Range Achievement Test-IV, Reading Subtest. SFS = Social Functioning scale (higher = better functioning); PANSS= Positive and Negative Syndrome scale; YMRS=Young Mania Rating scale; MADRS= Montgomery Asberg Depression Rating scale; IQR=interquartile range;
Medication class consistent with Hill et al. (2013) and Tamminga et al. (2013).
A diagnosis of schizophrenia was made at consensus meetings using all available information including findings from the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)(First et al., 1995). Healthy comparison subjects had no history of a psychotic disorder or recurrent depression, and no known immediate family history of these disorders. Exclusion criteria for all participants included: history of head injury with loss of consciousness >10 minutes; pregnancy; positive urine toxicology screen for common drugs of abuse on the day of testing; diagnosis of substance abuse in the past 30 days or substance dependence in the past 6 months; history of systemic medical or neurological disorder affecting mood or cognition; or age-corrected Wide-Range Achievement Test reading test standard score ≤70.
2.2 Serum hormone assays
Blood samples were drawn in the morning when possible (97% blood draws before noon). Samples were stored in plain tubes, spun at 4°C, divided into aliquots (300ul), and stored at −80°C. Samples were batched, diluted in an assay buffer to give reliable results within the linear portion of the standard curve (OT 1:4; AVP 1:2), and completed in duplicate using unextracted plasma. EIA kits (Enzo Life Sciences/Assay Designs) were used to quantify OT and AVP (Carter et al., 2007) and both hormones were assayed simultaneously. Samples were not extracted prior to performing the EIA as studies using mass spectrometry demonstrate high levels of OT in plasma and serum and indicate that methods involving extraction of samples removes much of this peptide (Brandtzaeg et al., 2016; MacLean et al., 2018). These EIAs are highly sensitive (minimal detection levels <12 pg/ml OT; 4 pg/ml AVP) and cross-reactivity between OT and AVP is low (<0.04%). All OT values >2000 pg/ml and AVP values >400 pg/ml were re-run to confirm sample accuracy. This was only the case for OT level; no cases had AVP values >400pg/ml. For both assays, intra-assay coefficients of variation were <10.7%. Hormone values were log transformed for statistical analysis. Mean daily chlorpromazine equivalent in patients was not associated with OT (r=−0.21, p=0.33) or AVP levels (r=−0.07, p=0.74). Age was associated with OT (r=0.23, p=0.03), but not AVP levels (r=0.13, p=0.23).
2.3. Emotion processing and cognition
From the Brief Assessment of Cognition in Schizophrenia (BACS) neuropsychological test battery, we selected three subtests including verbal learning (list learning), verbal fluency (word generation), and processing speed (symbol-coding). The Penn Emotion Recognition (ER)-40 Test assessed the ability to accurately recognize facial emotions (Gur et al., 2002). These tests were selected based on previous research indicating that the tests demonstrate sex differences in the general population (Kramer et al., 1988; Kramer et al., 1997; Mann, 1990; Weiss et al., 2003; Weiss et al., 2006; Williams et al., 2009) or if tests (verbal learning, processing speed) showed a sex difference in favor of women (p’s<0.01) in the large sample of healthy controls from the Bipolar and Schizophrenia Network on Intermediate Phenotypes study (N=304, 55% women).
2.4. Resting State Data Acquisition
Participants underwent 5 minutes of scanning using a GE Signa EXCITE 3.0 Tesla MR imaging system and an 8-channel phased array head coil. Participants fixated on a central crosshair for the duration of the scan. Video monitoring of participants eyes confirmed adherence to this instruction. Soft ear plugs were used to reduce scan noise, and head motion was minimized with head cushions. Echo-planar imaging sensitive to changes in BOLD signals (repetition time= 1775msec, echo time= 27msec, flip angle= 60°) were obtained. The slice thickness was 4 mm (1mm gap) with a matrix size of 64×64 and a field of view of 220×220 mm2, resulting in a voxel size of 3.44×3.44×5 mm3. Each brain volume was comprised of 29 axial slices, and each functional run contained 210 image volumes.
2.5. Data Processing
The first 5 volumes were discarded to achieve steady-state longitudinal magnetization. Remaining data were preprocessed by Data Processing Assistant for Resting-State fMRI, version 2.3 (http://rfmri.org/DPARSF) software, implemented within the MATLAB toolbox, including slice timing and head motion correction, spatial normalization, and smoothing with a Gaussian kernel of 6 mm3 full width at half maximum. fMRI images were spatially normalized to Montreal Neurological Institute space using the standard echo-planar imaging template after resampling the images to achieve a voxel resolution of 3×3×3 mm. The measurement of ALFF ranged from 0.01 to 0.08 Hz, which is believed to reflect regional spontaneous neural activity (Cordes et al., 2001). To achieve this, we set the bandpass filtering range as 0.01–0.08 HZ and removed the linear trend. Then, using fast Fourier transformation (parameters: taper percentage=0, fast Fourier transform length =shortest), we transformed the time series to the frequency domain, and obtained the power spectrum. Next, power was square root transformed and then averaged across 0.01–0.08 Hz to yield a measure of the ALFF from each voxel. Finally, the ALFF of each voxel was then divided by the global mean ALFF value of the individual to standardize data across subjects.
2.6. Statistical Analyses
All statistical analytic processes were performed in REST software (Resting-State fMRI Data Analysis Toolkit V1.8; http://restfmri.net/forum/rest)(Zang et al., 2007). First, we calculated the voxel-wise correlations between ALFF and hormone data within the patient and control groups respectively, and within male and female participants in each group, with age treated as a covariate. To detect differences in hormone-brain activity correlations between groups, voxel-based comparison of correlation maps was performed in SPM8 (http://www.fil.ion.ecl.ac.uk/spm). Primary analyses compared male patients and controls, and female patients and controls. The average correlation coefficient of brain regions (clusters of voxels) showing significant differences in hormone – brain physiology correlations were extracted, compared across groups, and correlated with clinical and demographic characteristics using cocor software (comparing correlations; http://comparingcorrelations.org). Primary correlational analyses investigated relations between hormones and brain physiology, and between brain physiology and behavior. A series of multivariable linear regressions were then conducted to determine whether brain physiology mediated hormone-behavior associations (Hafeman, 2009; Kaufman, 1999).
In all image analyses, we used Monte Carlo simulations and AlphSim software to correct for multiple comparisons when testing for statistical significance. The probability of a false-positive detection for the study was set to p<0.05 using a minimum cluster size of 5 contiguous voxels significant individually at a threshold of p<0.05 (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). The AlphSim calculation was conducted using REST software (http://restfmri.net/forum/index.php), which integrated the true smoothness kernel estimated based on the statistical map and mask file.
3. Results
AVP, but not OT levels, were lower in female patients compared to controls (p=0.001; Table 1). There was a trend for OT levels, but not AVP, to be lower in male patients compared to controls (p=0.07). Consistent with our previous publication (Rubin et al., 2014), OT levels were associated with facial emotion recognition in female controls (r=0.39, p=0.03) but not among any other group (p’s>0.07). There were also no associations between AVP levels and performance (p’s>0.15).
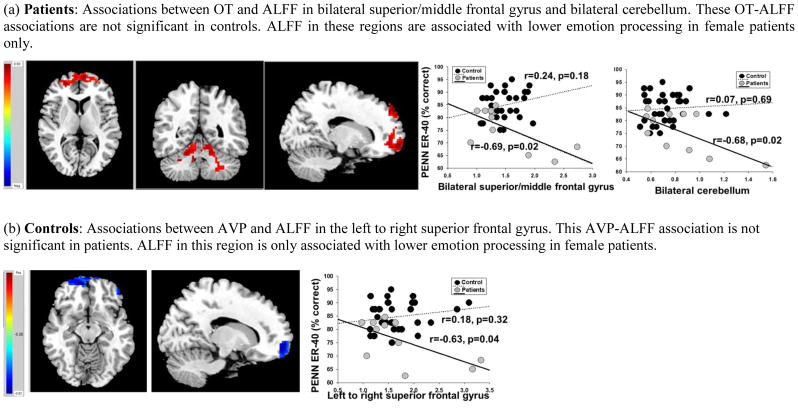
In women, lower OT levels were associated with lower ALFF in the right superior frontal gyrus, extending to the left superior frontal gyrus and bilateral middle frontal gyrus (r=0.71, p=0.01) and right anterior and posterior cerebellum, extending to left cerebellum in patients (r=0.69 p=0.01) but not controls (r=−0.09 and r=−0.14, respectively; Figure 1a). The degree to which OT levels were associated with ALFF in frontal and cerebellar cortices differed between female patients and controls (p=0.007 and p=0.01, respectively). In female patients, but not controls, higher ALFF in frontal and cerebellar cortices were associated with poorer facial emotion recognition (p’s<0.05; Figure 1a) and higher ALFF in cerebellar cortices was associated with poorer semantic fluency (r=−0.61, p=0.04). Mediational analyses indicated that OT levels influenced changes in facial emotion recognition through ALFF in the frontal cortex [69% of the total effect (65%) was explained by the indirect effect] and ALFF in the cerebellum [63% of the total effect (65%) was explained by the indirect effect]. OT levels influenced changes in semantic fluency through ALFF in the cerebellum [68% of the total effect (55%) was explained by the indirect effect]. In women, lower AVP levels were associated with higher ALFF in the superior frontal gyri in controls (r=−0.44, p=0.01) but not patients (r=0.21, p=0.53; Figure 1b). The degree to which AVP levels were differentially associated with ALFF in frontal cortex in patients and controls was at the trend level (p=0.07). In female patients, but not controls, ALFF in this region was significantly associated with poorer emotion processing (p=0.04; Figure 1b). Mediational analyses indicated that AVP levels had only minimal influences in emotion processing through ALFF the frontal cortex [23% of the total effect (94%) was explained by the indirect effect].
Figure 1. Neuroanatomical regions showing differences between female patients and healthy female controls in the degree to which peripheral oxytocin (OT) and vasopressin (AVP) are associated with amplitude of low-frequency fluctuations (ALFF) and ALFF- emotion processing associations.
Red indicates positive association whereas Blue indicates an inverse association.
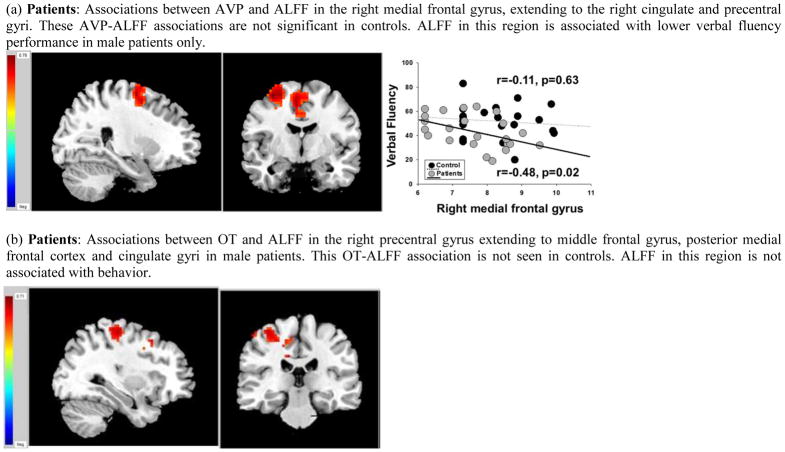
In men, lower AVP levels were associated with lower ALFF in the right medial frontal gyrus, extending to right cingulate and precentral gyri in patients (r=0.52, p=0.01) but not controls (r=−0.01, p=0.96; Figure 2a). The degree to which AVP levels were associated with ALFF in the medial frontal gyrus in male patients and controls was at the trend level (p=0.06). In male patients, but not controls, higher ALFF in the right medial frontal gyrus was associated with poorer performance on verbal fluency (p=0.02; Figure 2a). This pattern was seen for both letter (r=−0.39, p=0.06, and category fluency, r=−0.50, p=0.01). Mediational analyses indicated that AVP levels had some influence on letter fluency [56% of the total effect (22%) was explained by the indirect effect] and category fluency [32% of the total effect (43%) was explained by the indirect effect] through ALFF in frontal cortex.
Figure 2. Neuroanatomical regions showing differences between male patients and healthy male controls in the degree to which peripheral oxytocin (OT) and vasopressin (AVP) are associated with amplitude of low-frequency fluctuations (ALFF) and ALFF-cognition/emotion processing.
Red indicates positive association whereas Blue indicates an inverse association.
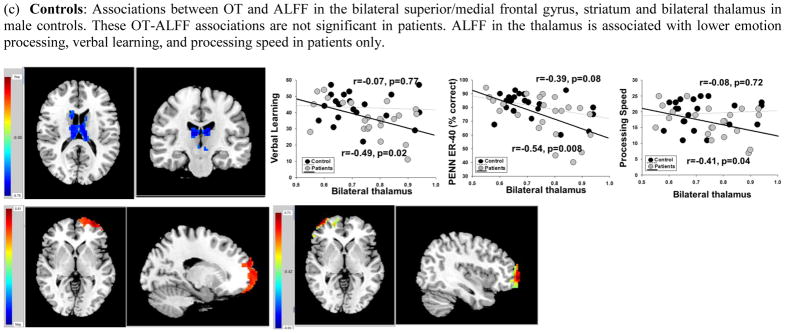
In men, lower OT levels were associated with lower ALFF in the right precentral gyrus, extending to middle frontal and cingulate gyri in male patients (r=0.48, p=0.02) but not controls (r=0.10, p=0.66; Figure 2b). Higher OT levels were associated with higher ALFF in bilateral superior/medial frontal gyrus (left hemisphere r=0.54, p=0.009; right hemisphere r=0.47, p=0.02) and lower ALFF in the thalamus bilaterally (r=−0.51, p=0.01) in controls but not patients (r =0.15, r=0.004, r=−0.03, respectively). The degree to which OT was associated with these regions between male patients and controls was at the trend level in the thalamus (p=0.06). The differences in associations between the two groups in the other regions had p-values ranging between 0.10 and 0.17. In male patients, but not controls, higher ALFF in the thalamus was associated with poorer emotion recognition (r=−0.54, p=0.008), verbal learning (r=−0.49, p=0.02), and processing speed (r=−0.41, p=0.05). Mediational analyses indicated that OT levels had some influence on emotion recognition, verbal learning, and processing speed through ALFF in the thalamus [46% of the total effect (16%), 92% of the total effect (7%), and 53% of the total effect (13%), respectively). Controlling for age did not change the pattern of brain physiology-behavior associations in any group.
4. Discussion
To our knowledge, there have been no previous neuroimaging investigations evaluating how physiologic levels of OT and AVP may influence brain physiology in women and men with schizophrenia. In this preliminary investigation, we identified sex-specific hormonal alterations (e.g., lower AVP in female patients vs. female controls) and novel sex-specific associations of neurohormone levels with regional resting state brain activity. Brain activity in areas where these associations were detected were differentially associated with cognitive function and emotion processing in male and female patients. While further work is needed to determine mechanisms for these findings, findings of brain physiology-behavior associations in schizophrenia patients and lack of associations in controls could indicate that neuroendocrine levels and in some cases abnormalities may disrupt typical brain-behavior relations. In other cases, endocrine levels may interact with illness-related neuroanatomic alterations in ways that lead to disruptions or compensatory changes in resting neurophysiological activity and behavior. Overall, our findings suggest that peripheral OT and AVP levels may contribute to sex differences in brain and behavioral functions in individuals with schizophrenia.
In general, brain regions where spontaneous brain activity was associated with basal OT and AVP levels within each patient group (e.g., female patients, male patients) are consistent with areas of structural or functional alterations seen in previous schizophrenia studies. Although we did not find the amygdala activity to be associated with OT, numerous studies demonstrate the involvement of other brain regions that are sensitive to exogenous OT (Bethlehem et al., 2013). Despite large number of studies examining the effects of exogenous OT administration on task-based fMRI (Tully et al., 2018; Wang et al., 2017), fewer studies have examined the impact of OT administration on the resting state of the brain and the majority have focused on the amygdala and its functional correlates. Studies examining the impact of OT administration on resting state fMRI have shown an effect on amygdala coupling with the medial prefrontal cortex, middle frontal and superior frontal gyri, anterior cingulate, and cerebellum (Ebner et al., 2016; Eckstein et al., 2017; Sripada et al., 2013). Our findings investigating associations with intrinsic hormone levels are consistent with that literature in that basal OT levels were generally associated with activity of frontal regions (middle and superior frontal), cingulate cortex, cerebellum, and thalamus. Fewer studies have investigated the impact of AVP administration on task-based fMRI (Chen et al., 2016; Lee et al., 2013; Rilling et al., 2014). In general, these studies demonstrate that AVP administration can have effects on temporal-limbic and insula activity which we did not see in the present study. However, our previous study examining basal AVP levels in relation to resting state fMRI in healthy individuals did demonstrate associations between AVP and prefrontal regions (Rubin et al., 2017). Here we also saw basal AVP levels to be associated with activity in some frontal regions (middle, medial, and superior frontal gyri) and cingulate cortex.
In the present study, patterns of associations between OT, AVP, and resting state brain physiology differed in women and men with schizophrenia compared to sex-matched controls. We have previously demonstrated associations between these hormones and resting brain physiology in healthy individuals (Rubin et al., 2017), and our current findings suggests that these associations are different in male and female patients. This suggests that these hormones differentially modulate brain networks important for cognition and emotion processing in women and men with schizophrenia. Among female patients, we found lower levels of AVP but not OT. This hormone profile is consistent with many (Rubin et al., 2014; Rubin et al., 2010; Rubin et al., 2015; Rubin et al., 2018), but not all (Rubin et al., 2018), of our previous studies. Furthermore, among female patients, there were some hormone-brain physiology associations that suggested a loss of hormonal modulation such as the association of AVP and ALFF in frontal cortex (superior frontal gyri), while other findings suggested increased hormonal modulation such as the association of OT and ALFF in frontal (superior frontal/middle frontal gyri) and cerebellar cortices even though OT levels were not themselves abnormal.
Previous studies have reported lower AVP levels in mixed samples of men and women with chronic schizophrenia (Rubin et al., 2014), and that frontal (superior/middle frontal) and cerebellar cortex show cerebral functional deficits among individuals with schizophrenia that are related to cognition (Giraldo-Chica and Woodward, 2017; Lui et al., 2009; Lui et al., 2015; Sheffield and Barch, 2016). Moreover, functional alterations of frontal and cerebellar cortex are among the most commonly reported regional abnormalities in schizophrenia during task-based fMRI studies targeting emotional face processing (Fusar-Poli et al., 2009). These hormones may be less involved in cognitive function as there was only one brain region (cerebellum) associated with hormone levels that were also associated with cognitive performance (fluency) among women with schizophrenia in the present study.
The biological mechanisms for these findings appear to be complex. One possibility is that the AVP but not the OT pathway may be disrupted, and thus OT may have a compensatory role and be more involved in associations with resting brain physiology regions important for emotion processing in women with schizophrenia. Alternatively it is possible that females are generally more dependent on OT than AVP (Rubin et al., 2017) particularly in brain regions important for emotion processing. For example, in socially monogamous prairie voles, females have higher densities of OT receptors than males in prefrontal regions (Smeltzer et al., 2006). To date, it has yet to be determined if exogenous OT may be beneficial for women with schizophrenia in improving emotion processing, in contrast to some studies with male patients.
In contrast to our findings in female patients, there was a non-significant trend for male patients to show lower levels of OT but not AVP compared to male controls. Others have demonstrated lower OT levels in male patients compared to male controls (Jobst et al., 2014). Additionally, among male patients, there were some hormone-brain physiology associations that suggested reduced hormonal modulation such as that of OT on ALFF in frontal cortex (superior/medial frontal) and thalamus, while other findings suggested increased hormonal modulation involving both hormones and ALFF in frontal cortex (middle frontal gyrus/cingulate). The brain regions where activity was associated with hormones in male patients have also been shown to have functional deficits among individuals with schizophrenia (Lui et al., 2015). Individuals with schizophrenia demonstrate abnormal neural network function within and between thalamo-cortical loops compared to healthy controls (Giraldo-Chica and Woodward, 2017; Lui et al., 2009; Lui et al., 2015; Sheffield and Barch, 2016). Additionally, in male patients, ALFF in the medial frontal gyrus was associated with verbal fluency, and ALFF in the thalamus was associated with lower emotion processing, processing speed, and verbal learning. In contrast to women with schizophrenia, findings suggest that in men with schizophrenia the OT pathway but not the AVP pathway may be altered. However, both hormones appear to be involved in associations with resting brain physiology regions important for both cognitive and emotion processing deficits in men with schizophrenia. Understanding the interaction between OT and brain function may provide fundamental clues to mechanisms underlying the sex differences and individual differences in complex cognitive and emotional deficits (Carter, 2017).
The present study has limitations including a cross-sectional study design, single measurement of hormone levels, absence of measurement of plasma osmolality/sodium which facilitates interpretation of AVP levels, and small number of participants for study comparing sex differences in the two participant groups. Additionally, the relevance of peripheral measures (measured here) to central nervous system function and behavior are a common issue raised in psychiatric neuroendocrinology. Peripheral measures are imperfect markers of central hormone levels, but animal studies demonstrate that central and peripheral hormone levels are meaningfully associated (Landgraf and Neumann, 2004). Additionally, other tests of cognition and emotion processing may yield different findings than observed in the present study. Finally, a 5-minute resting state scan was used and longer sequences have more recently become preferred for more precise estimations of resting state function and connectivity. We note that the data included for analysis passed stringent thresholds for quality ensuring there was adequate data for detecting effects observed in the study. The 5-minute sequence with similar quality checking has been suitable to inform a variety of types of information (Lui et al., 2015; Meda et al., 2016; Meda et al., 2014; Meda et al., 2015; Rubin et al., 2017). Despite these limitations, our findings provide evidence that the interplay between neuroendocrine systems, neural networks, and behavior may contribute to sex differences in schizophrenia. Specifically, associations with frontal and cerebellar cortices in female patients and the posterior cingulate in male patients may play sexually dimorphic roles in emotion processing and cognitive deficits in schizophrenia.
Acknowledgments
Role of the funding source
The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
This work was supported in part by a 2012 NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation to Dr. Rubin, and by the National Institute of Health (K12HD055892, K08MH083888, MH083126, MH077851, MH078113, MH077945, MH077852, and MH077862), the National Natural Science Foundation of China (Grant No. 81371527), and the Program for Changjiang Scholars and Innovative Research Team in Universities of China.
Footnotes
Contributors
Drs. Rubin and Sweeney conceived the neuroendocrine study idea. Dr. Sweeney coordinated the Chicago B-SNIP study and the sample presented here was ascertained as part of the B-SNIP study at the Chicago site. Drs. Rubin, Li, Yao, Lui, Carter, Drogos, Sweeney, and Keedy wrote the first draft of the manuscript. Dr. Rubin and Dr. Yao and Li take responsibility for the integrity of the hormone data and the MRI data analyses respectively. Drs. Yao, Li, and Lui, planned and conducted the resting state analyses. Dr. Sweeney provided oversight as principal investigator of the study. Dr. Carter developed the methodology to examine oxytocin and vasopressin, and assays were done in her laboratory by Dr. Pournajafi-Nazarloo and Dr. Lauren Drogos. Dr. Bishop collected and managed the biological samples. All authors contributed to the writing of the manuscript and approved the final version.
Conflicts of Interest
Dr. Tamminga reports the following financial disclosures: American Psychiatric Association, Deputy Editor; Astellas, Ad Hoc Consultant; Autifony, Ad Hoc Consultant; Intra-cellular Therapies (ITI), Advisory Board, drug development; Pfizer, Ad Hoc Consultant; Sunovion, Investigator Initiated grant funding. Investigator Initiated grant funding. The remaining authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38(7):962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, Leknes S, Lundanes E, Wilson SR. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep. 2016;6:31693. doi: 10.1038/srep31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. The Oxytocin-Vasopressin Pathway in the Context of Love and Fear. Front Endocrinol (Lausanne) 2017;8:356. doi: 10.3389/fendo.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain imaging and behavior. 2016;10(2):581–593. doi: 10.1007/s11682-015-9411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology. 2016;69:50–59. doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Markett S, Kendrick KM, Ditzen B, Liu F, Hurlemann R, Becker B. Oxytocin differentially alters resting state functional connectivity between amygdala subregions and emotional control networks: Inverse correlation with depressive traits. Neuroimage. 2017;149:458–467. doi: 10.1016/j.neuroimage.2017.01.078. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63. doi: 10.1016/j.schres.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM. Sex and brain abnormalities in schizophrenia: fact or fiction? Harv Rev Psychiatry. 1996;4(2):110–115. doi: 10.3109/10673229609030533. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS, Jr, Faraone SV, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59(2):154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Walder DJ. Sex differences in schizophrenia: The case for developmental origins and etiological implications. In: Sharma T, Harvey P, editors. The Early Course of Schizophrenia. Oxford University Press; Oxford, United Kingdom: 2006. pp. 147–173. [Google Scholar]
- Grigoriadis S, Seeman MV. The role of estrogen in schizophrenia: implications for schizophrenia practice guidelines for women. Can J Psychiatry. 2002;47(5):437–442. doi: 10.1177/070674370204700504. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler C, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry. 2004;55(5):512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Hafeman DM. “Proportion explained”: a causal interpretation for standard measures of indirect effect? Am J Epidemiol. 2009;170(11):1443–1448. doi: 10.1093/aje/kwp283. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170(11):1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst A, Dehning S, Ruf S, Notz T, Buchheim A, Henning-Fast K, Meissner D, Meyer S, Bondy B, Muller N, Zill P. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2014;26(6):347–355. doi: 10.1017/neu.2014.20. [DOI] [PubMed] [Google Scholar]
- Kaufman JS. Progress and pitfalls in the social epidemiology of cancer. Cancer causes & control: CCC. 1999;10(6):489–494. doi: 10.1023/a:1008958104748. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44:907–915. [Google Scholar]
- Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11(4):577–584. doi: 10.1037//0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, Davis JM, Morris JP, Connelly JJ. Plasma oxytocin explains individual differences in neural substrates of social perception. Frontiers in human neuroscience. 2015;9:132. doi: 10.3389/fnhum.2015.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Coccaro EF, Cremers H, McCarron R, Lu SF, Brownstein MJ, Simon NG. A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: an fMRI study. Frontiers in systems neuroscience. 2013;7:100. doi: 10.3389/fnsys.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L, Tang H, Zhou XJ, Mechelli A, Collier DA, Sweeney JA, Li T, Gong Q. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166(2):196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- Lui S, Yao L, Xiao Y, Keedy SK, Reilly JL, Keefe RS, Tamminga CA, Keshavan MS, Pearlson GD, Gong Q, Sweeney JA. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45(1):97–108. doi: 10.1017/S003329171400110X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Gesquiere LR, Gee N, Levy K, Martin WL, Carter CS. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J Neurosci Methods. 2018;293:67–76. doi: 10.1016/j.jneumeth.2017.08.033. [DOI] [PubMed] [Google Scholar]
- Mann VA, Sasanuma S, Sakuma N, Masaki S. Sex differences in cognitive abilities: A cross-cultural perspective. Neuropsychologia. 1990;28(10):1063–1077. doi: 10.1016/0028-3932(90)90141-a. [DOI] [PubMed] [Google Scholar]
- Meda SA, Clementz BA, Sweeney JA, Keshavan MS, Tamminga CA, Ivleva EI, Pearlson GD. Examining Functional Resting-State Connectivity in Psychosis and Its Subgroups in the Bipolar-Schizophrenia Network on Intermediate Phenotypes Cohort. Biological psychiatry Cognitive neuroscience and neuroimaging. 2016;1(6):488–497. doi: 10.1016/j.bpsc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Meda SA, Ruano G, Windemuth A, O’Neil K, Berwise C, Dunn SM, Boccaccio LE, Narayanan B, Kocherla M, Sprooten E, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Calhoun VD, Pearlson GD. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111(19):E2066–2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Wang Z, Ivleva EI, Poudyal G, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Schretlen DJ, Calhoun VD, Lui S, Damaraju E, Pearlson GD. Frequency-Specific Neural Signatures of Spontaneous Low-Frequency Resting State Fluctuations in Psychosis: Evidence From Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Consortium. Schizophr Bull. 2015;41(6):1336–1348. doi: 10.1093/schbul/sbv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, Ruocco AC, Keedy SK, Reilly JL, Keshavan MS, Pearlson GD, Tamminga CA, Gershon ES, Sweeney JA. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr Bull. 2014;40(6):1374–1384. doi: 10.1093/schbul/sbu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Harris MS, Hill SK, Reilly JL, Sweeney JA. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr Res. 2013;146(1–3):138–143. doi: 10.1016/j.schres.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res. 2011;130(1–3):266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Effects of sex, menstrual cycle phase, and endogenous hormones on cognition in schizophrenia. Schizophr Res. 2015;166(1–3):269–275. doi: 10.1016/j.schres.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Wehring HJ, Demyanovich H, Sue Carter C, Pournajafi-Nazarloo H, Feldman SM, Earl AK, August S, Gold JM, Kelly DL. Peripheral oxytocin and vasopressin are associated with clinical symptom severity and cognitive functioning in midlife women with chronic schizophrenia. Schizophr Res. 2018;195:409–411. doi: 10.1016/j.schres.2017.09.041. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Yao L, Keedy SK, Reilly JL, Bishop JR, Carter CS, Pournajafi-Nazarloo H, Drogos LL, Tamminga CA, Pearlson GD, Keshavan MS, Clementz BA, Hill SK, Liao W, Ji GJ, Lui S, Sweeney JA. Sex differences in associations of arginine vasopressin and oxytocin with resting-state functional brain connectivity. J Neurosci Res. 2017;95(1–2):576–586. doi: 10.1002/jnr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108–120. doi: 10.1016/j.neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou XJ, Xu XJ, Zeng XZ, Liu Y, Yuan HS, Xing Y, Jia MX, Wei QY, Han SP, Zhang R, Han JS. A Volumetric and Functional Connectivity MRI Study of Brain Arginine-Vasopressin Pathways in Autistic Children. Neurosci Bull. 2017;33(2):130–142. doi: 10.1007/s12264-017-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol. 2013;16(2):255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tully J, Gabay AS, Brown D, Murphy DGM, Blackwood N. The effect of intranasal oxytocin on neural response to facial emotions in healthy adults as measured by functional MRI: A systematic review. Psychiatry Res. 2018;272:17–29. doi: 10.1016/j.pscychresns.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yan X, Li M, Ma Y. Neural substrates underlying the effects of oxytocin: a quantitative meta-analysis of pharmaco-imaging studies. Social cognitive and affective neuroscience. 2017;12(10):1565–1573. doi: 10.1093/scan/nsx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Kemmler G, Deisenhammer EA, Fleischhacker WW, Delazer M. Sex differences in cognitive function. Pers Individ Dif. 2003:863–875. [Google Scholar]
- Weiss EM, Ragland JD, Brensinger CM, Bilker WB, Deisenhammer EA, Delazer M. Sex differences in clustering and switching in verbal fluency tasks. J Int Neuropsychol Soc. 2006;12(4):502–509. doi: 10.1017/s1355617706060656. [DOI] [PubMed] [Google Scholar]
- Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. J Clin Exp Neuropsychol. 2009;31(3):257–277. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]