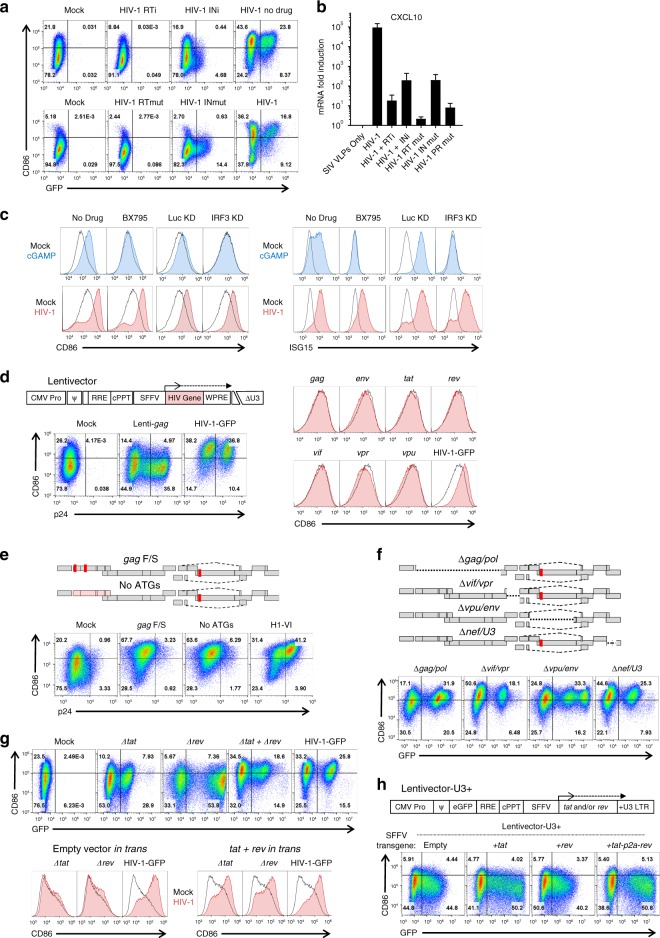
Fig. 2.
Native HIV-1 RNA regulation is necessary for DC maturation. a Assessment of GFP and CD86 by flow cytometry following transduction with, top, HIV-1-GFP in the presence of 5 µM nevirapine (RTi), 10 µM raltegravir (INi), or no drug, and, bottom, HIV-1-GFP bearing mutant RT-D185K/D186L (RTmut) or mutant IN-D116A (INmut). b qRT-PCR quantitation of CXCL10 mRNA from the same DCs as a. c DCs treated with 1 µM of the TBK1 inhibitor BX795, or expressing shRNAs targeting either IRF3 or luciferase control16, were challenged with 25 µg/mL cGAMP or HIV-1-GFP and assayed by flow cytometry for CD86 and ISG15. d Flow cytometry of DCs after transduction with minimal lentivectors expressing codon optimized HIV-1 genes; e, HIV-1-GFP in which translation was disrupted by two frameshifts in gag or by mutation of the first 14 AUGs in gag; f, HIV-1-GFP bearing deletion mutations encompassing gag/pol, vif/vpr, vpu/env, or nef/U3-LTR; g, HIV-1-GFP bearing mutations in tat or rev, co-transduced with both mutants, or co-transduced with minimal vector expressing tat and rev in trans; or h minimal lentivector with GFP in place of gag, SFFV promoter driving expression of tat, rev, or both, and repaired U3 in the 3′ LTR. When an essential viral component was disrupted within HIV-1-GFP, the factor in question was provided in trans, either during assembly in transfected HEK293 cells, or within transduced DCs, as appropriate (see Methods). Shown are blood donor data representative of n = 6 (a, b, e, f), n = 12 (c, g, h), n = 8 (d). To determine significance, the MFI of individual flow cytometry samples was calculated as fold-change versus control. When data from each donor replicate within a experiment were combined, the difference in MFI for all experimental vs control conditions was significant in all cases, p < 0.01; one-way ANOVA, Dunnett’s post-test against HIV-1-GFP for a, c, d, h or lentivector control for e, f. qRT-PCR data are mean±SD (p < 0.0001; two-way ANOVA, Dunnett’s post-test)