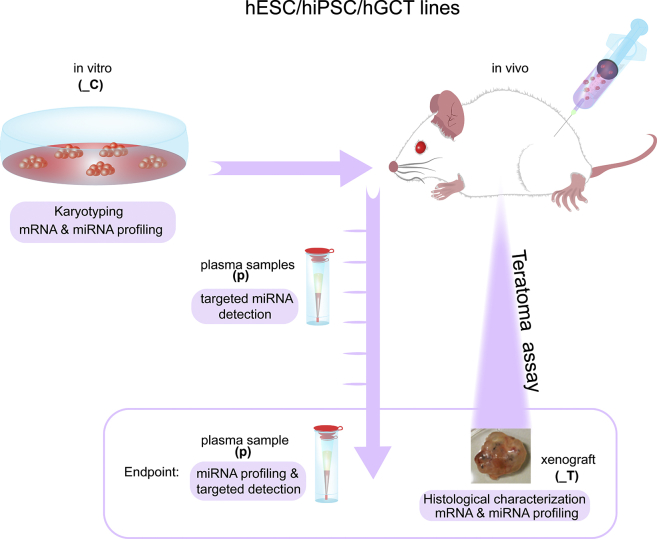
Figure 1.
Schematic Representation of the Work Flow Performed
hPSC (iPSCs and hESCs) and hGCT cell lines were injected subcutaneously into immunodeficient mice (teratoma assay). Cell lines were karyotyped before injection and collected for mRNA and miRNA analysis. During the teratoma assay, blood samples for plasma preparation were timely collected until the tumors reached the maximum allowed size of 2 cm3 (endpoint). At the endpoint, xenografts were collected for histological characterization, and for mRNA and miRNA isolation, and blood samples for plasma isolation (_C, cell lines; _T, xenografts; and p, plasma samples). The mRNA expression profile of the xenograft derived from hPSC and hGCT lines was compared with a set of hGCTs from patients (Table S2). A list of all abbreviations used in the study is given in Table S1A.