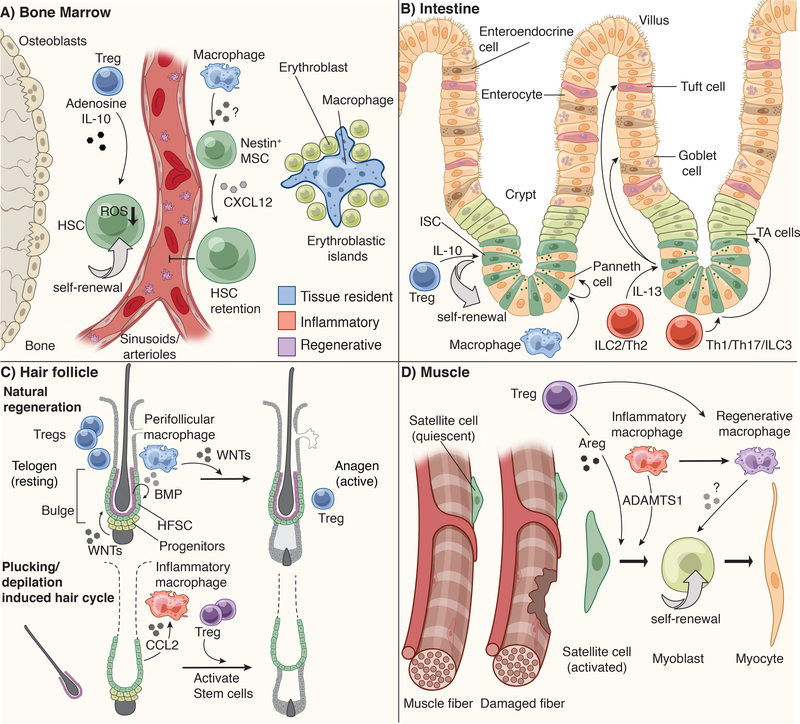
Figure 3: Niche specific immune-stem cell interactions.
A) Hematopoietic stem cells (HSCs) reside in the bone marrow (BM) in close apposition of sinusoids and arterioles. They are retained in this niche by Nestin+ mesenchymal stem cell (MSC)-derived CXCL12. Bone marrow macrophages are critical for maintaining the production of CXCL12 and retaining HSCs in this niche. Bone marrow Tregs are also localized close to HSCs and produce IL-10 and Adenosine to control HSC quiescence and pool size. In the absence of Tregs, HSCs produce more reactive oxygen species (ROS) and consequently increase their numbers. Erythroblast progenitors in the BM aggregate around a central macrophage, which provides cues to direct their maturation into erythrocytes.
B) Intestinal stem cells (ISCs) reside in crypt structures at the base of intestinal villi and are flanked by Paneth cells and transit amplifying (TA) cells. ISCs give rise to all intestinal lineages, including goblet cells, enteroendocrine cells, tuft cells, enterocytes and Paneth cells. However, when ISCs at the base of the crypt are ablated, these progenitors can replenish niche vacancies. Intestinal macrophages promote the differentiation of ISCs to Paneth cells, while resident Tregs promote ISC self-renewal via IL-10. CD4+ T helper (Th)1 and Th17 cells and innate lymphoid cells (ILC) type 3 promote the production of transit amplifying progeny, while Th2 and ILC2 promote differentiation of tuft and goblet cells via IL-13.
C) The hair follicle is one of the few systems in mammals where tissue regeneration happens in natural bursts (the hair cycle) and in the absence of injury. The bulge region of the hair follicle is home to hair follicle stem cells (HFSCs). At the bulge base (hair germ), ‘primed stem cells’ give rise to the short-term progenitors that undergo natural cyclical bouts of active tissue regeneration (anagen). Growth inhibitory signals such as bone morphogenic proteins (BMPs), supplied by both the dermis and the inner bulge niche layer, keep stem cells in quiescence throughout most of the hair cycle. In the resting phase of the hair cycle (telogen), WNT signals and BMP inhibitor levels in the hair germ accumulate until stem cells become activated to launch a new tissue regenerative cycle.
Perifollicular macrophages are a crucial source of WNTs and tip the balance in favor of regeneration. By contrast, Tregs are highest in telogen when stem cells are at the height of quiescence; Tregs reach a low point at the height of the hair growth phase. Hair plucking removes the inner bulge and its quiescence signals, thereby precociously inducing anagen. During this injury response, inflammatory macrophages are also found near the bulge, where they appear to promote hair cycling and disseminate distress signals to adjacent unplucked follicles. Regenerative Tregs also appear to facilitate the progression to anagen following hair depilation.
D) Muscle stem cells (satellite cells) are quiescent at steady state and only activate following injury. Regenerative Tregs and inflammatory macrophages (M1) support satellite cell self-renewal and activation via amphiregulin (Areg) and ADAMTS1, respectively. As repair progresses, Tregs facilitate the switch from inflammatory M1 to regenerative M2 macrophages, which promote myoblast self-renewal and differentiation.