Abstract
As a potent macrolide immunosuppressant, cyclosporine A (CsA) is used to treat multiple autoimmune diseases, including non-autoimmune and autoimmune-mediated dry eye disease, rheumatoid arthritis and psoriasis. Despite its potency, CsA has poor solubility, poor bioavailability, and can cause serious adverse reactions such as nephrotoxicity and neurotoxicity. To overcome these limitations, we invented a new strategy to carry CsA by fusing its cognate human receptor, cyclophilin A (CypA), to a 73 kDa elastin-like polypeptide (ELP) termed A192 using recombinant protein expression. Derived from human tropoelastin, ELPs are characterized by the ability to phase separate above a temperature that is a function of variables including concentration, molecular weight, and hydrophobicity. The resultant fusion protein, termed CA192, which assembles into a dimeric species in solution, effectively binds and solubilizes CsA with a Kd of 189 nM, comparable to that of endogenous CypA with a Kd of 35.5 nM. The release profile of CsA from CA192 follows a one phase decay model with a half-life of 957.3h without a burst release stage. Moreover, CA192-CsA inhibited IL-2 expression induced in Jurkat cells through the calcineurin-NFAT signaling pathway with an IC50 of 1.2 nM, comparable to that of free CsA with an IC50 of 0.5 nM. The intravenous pharmacokinetics of CA192 followed a two-compartment model with a mean residence time of 7.3 hr. Subcutaneous administration revealed a bioavailability of 30% and a mean residence time of 15.9 hr. When given subcutaneously for 2 weeks starting at 14 weeks in male non-obese diabetic NOD mice, a model of autoimmune dacryoadenitis used to study Sjögren’s syndrome (SS), CA192-CsA (2.5 mg/kg, every other day) significantly (p=0.014) increased tear production relative to CA192 alone. Moreover, CA192 delivery reduced indications of CsA nephrotoxicity relative to free CsA. CA192 represents a viable new approach to deliver this effective but nephrotoxic agent in a modality that preserves therapeutic efficacy but suppresses drug toxicity.
Keywords: Sjögren’s Syndrome, Cyclosporine A, Dry Eye, Elastin-like Polypeptide, Inflammation, Interleukin-2
1. Introduction
Cyclosporine A (CsA) is a well-known lipophilic cyclic immunosuppressant peptide composed of 11 amino acids which works by blocking T-cell proliferation and inhibiting the release of inflammatory cytokines such as interleukin-2 (IL-2) and interferon gamma (IFN-γ) [1]. Through binding to its cognate receptor, cyclophilin A (CypA), CsA can inhibit the calciumcalmodulin activated phosphatase, calcineurin [2], making it a powerful tool in the available arsenal of immunomodulatory therapies. Mechanistically, the nuclear factor of activated T cells (NFAT), the inducible factor that binds the IL-2 promotor in activated T-cells [3], is dephosphorylated by activated calcineurin, which leads to its nuclear translocation and the induction of NFAT-mediated gene transcription of IL-2. When calcineurin phosphatase activity is inhibited, IL-2 gene expression and secretion are markedly reduced, thus generating the principal therapeutic effect of CsA. In addition to IL-2, other pro-inflammatory cytokines, such as IL-3, IL-4, IL-5, TNF-α and IFN-γ can also be downregulated by CsA [4].
Due to its immunosuppressive effects, CsA has been widely used to prevent rejection after organ transplantation and in modulation of inflammatory responses in autoimmune disorders including rheumatoid arthritis and psoriasis [5]. However, when administered systemically, CsA can lead to a number of serious adverse drug reactions (ADRs) because of its narrow therapeutic window [6]. Below the therapeutic window, CsA cannot effectively inhibit T cell proliferation and release of inflammatory cytokines required for its therapeutic actions, while above the therapeutic window, it may elicit severe side effects. In fact, though CsA has potential through modulation of IL-2 and other inflammatory cascades to treat a variety of autoimmune and inflammatory disorders, its clinical usage has been greatly limited due to the side effects including nephrotoxicity [7], hepatotoxicity [8], neurotoxicity [2], and hypertension [9]. In addition, due to its low solubility (27 μg/mL), CsA is usually formulated with polyoxyethylated castor oil (Cremophor EL®) for parenteral administration which can cause anaphylactoid reactions. When administrated topically, CsA has also been broadly used to treat dry eye syndrome (DES), a multifactorial disease of the ocular surface associated with decreased tear production and affecting an estimated 5–30% of the population [10, 11], presumably by suppressing ocular surface inflammation. Because of its hydrophobic properties, the only commercially-available form for topical administration of CsA is an oil-in-water emulsion, which has led to poor ocular tolerance, low bioavailability and instability [12].
Our goal in this study was to reformulate CsA for systemic delivery in a way that might reduce its systemic toxicity as well as minimize the frequency of administration while retaining its efficacy. We chose an autoimmune dry eye disease, Sjögren’s syndrome (SS), with systemic manifestations in the tear-producing lacrimal gland (LG) to test our reformulated construct. SS is a chronic autoimmune inflammatory disorder characterized by lymphocytic infiltration of exocrine glands, particularly LG and salivary glands (SG), and affecting more than 4 million Americans [13]. The hallmark clinical symptoms of SS are persistent dry eye and dry mouth, which eventually lead to severe corneal damage and compromised oral health. Disease progression is also associated with the development of constitutional symptoms involving pulmonary, neurological, vascular, and renal systems [14]. The LG and ocular surface system collectively represent an ideal disease model for evaluation of CsA efficacy, as SS is associated both with autoimmune-mediated LG and systemic inflammation as well as the reduced tear flow characteristic of aqueous-deficient DES, for which topical CsA is prescribed clinically. The well-established murine model for SS that we have used is the male Non-obese Diabetic (NOD) mouse [15], which spontaneously develops autoimmune dacryoadenitis (inflammation of the LG) and ocular surface dryness that recapitulates that seen in human SS [16, 17]. Although SS is more prevalent in women, utilizing the males instead of females in this murine model is based on their different patterns of disease development. Male NOD mice develop an early, profound lymphocytic infiltration of the LG at 8–12 weeks of age but exhibit little SG inflammation [18]. Female NOD mice develop a profound SG lymphocytic infiltration by 16–20 weeks of age, but lesser LG inflammation [19–21]. In addition to lymphocytic infiltration in the LG and reduced tear secretion, male NOD mice recapitulate other characteristics of human SS including expression of elevated matrix metalloproteinases (MMPs) in LG and in tears [22], and increased LG and tear levels of pro-inflammatory cytokines such as IL-1α, IL-2, IFN-γ, IL-6, and TNF-α [23].
The novel formulation of CsA developed here utilizes elastin-like polypeptides (ELPs). ELPs, derived from human tropoelastin, consist of pentameric repeats of (Val-Pro-Gly-Xaa-Gly)n where Xaa is the guest residue and n is the length of the repetitive units. ELPs have a unique inverse transition behavior. Below their transition temperature (Tt), they are highly water soluble but once the temperature rises above their Tt, ELPs undergo a phase separation process and self-assemble into different kinds of coacervates which can include particles of different sizes [24]. This phase separation is a fully reversible process and can be used to effectively purify ELP-conjugated materials [15]. Phase behavior can be precisely controlled by adjusting the hydrophobicity of guest residue “Xaa” and the number of pentapeptide repeats “n” [25].
Here we report that through molecular cloning, we have fused the cytosolic sequence of the human receptor of CsA, cyclophilin A (CypA), to a particular ELP, A192, which has the amino acid sequence of G(VPGAG)192Y. This CypA-A192 (CA192) fusion protein was designed to help solubilize CsA and to function as a drug carrier to improve the CsA safety profile when administered systemically. The results reported herein describes the biophysical properties of this protein-based carrier, as well as its high affinity for CsA, which significantly extended the in vitro release profile of CsA and, more importantly, the systemic circulation time upon parenteral administration. Furthermore, this study demonstrates that CsA bound to CA192 exhibits comparable activity in inhibition of IL-2 release in vitro in an activated Jurkat cell system relative to free CsA through the calcineurin-NFAT signaling pathway. Finally, in a proof-of-concept in vivo study, our results show that CsA bound to CA192 injected subcutaneously into male NOD mice with established disease improves tear flow and reduces systemic toxicity, relative to free CsA.
2. Materials and Methods
2.1. Reagents
NHS-rhodamine (46406), Hoechst 33342 (R37605), Lysotracker green (L7526), RIPA lysis and extraction buffer (89900), Protease and Phosphatase Inhibitor Cocktail (78440), dialysis cassette (20K MWCO, 3 mL) (66003) and Zeba Desalting Chromatography Cartridges (7K MWCO, 5 mL) (89935) were purchased from Thermo Fisher Scientific (Waltham, MA). Fetal Bovine Serum (FBS) (S11150H) was from Atlanta Biologicals, Inc. (Flowery Branch, GA). Penicillin-Streptomycin (10,000 U/mL) (1647113), advanced RPMI 1640 (1897242) and 1M HEPES (15630080) were purchased from Gibco® (Carlsbad, CA). Dulbecco’s Modified Eagle’s medium (DMEM) with L-glutamine (30–2002) was from ATCC® (Manassas, VA). Human serum albumin (A9511), Poly-D-lysine hydrobromide (P0899), phorbol 12-myristate 13-acetate (PMA) (P8139) and ionomycin (I0634) were from Sigma-Aldrich Corporation (St. Louis, MO). NFAT1 primary antibody (4389) and NFAT2 (8032) primary antibody and anti-rabbit IgG, HRP-linked secondary antibody (7074) were generated by Cell Signaling Technology (Danvers, MA). ProSignal Dura components (20–301) were purchased from Genesee Scientific (San Diego, CA). The Zone-Quick™ Diagnostic Threads were from Oassi Medical Inc. (Glendora, CA). Syringe filters (25 mm, 0.2 μM) (4192) were from Pall Corporation (Port Washington, NY).
2.2. Mice
Male NOD mice were bred at USC vivarium from breeding pairs obtained from Taconic (Hudson, NY). Male BALB/c mice (Stock No: 000651) were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal use was in compliance with protocols approved by the University of Southern California Institutional Animal Care and Use Committee, and experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.3. ELP biosynthesis and biophysical characterization
The encoding sequence of CypA was designed using Escherichia coli (E. coli) biased codons. The custom encoding sequence comprising the full length human CypA sequence was flanked by restriction recognition sites for NdeI and BamHI at the 5’ and 3’ ends to enable the insertion of the CypA sequence into the pET-25b(+) vector. Another BseRI restriction site was placed immediately ahead of the BamHI restriction site, allowing the ligation of the A192 encoding sequence, which was synthesized by recursive directional ligation in a modified pET-25b(+) vector [26]. Thus, the custom encoding sequence indicated below was ordered from Integrated DNA Technologies (IDT): 5’-CATATGGTTAACCCGACCGTTTTCTTCGACATCGCTGTTGACGGTGAACCGCTGGGTCGGTTTCTTTCGAACTGTTCGCTGACAAAGTTCCGAAAACCGCTGAAAACTTCCGTGCTCTGTCTACCGGTGAAAAAGGTTTCGGTTACAAAGGTTCTTGCTTCCACCGTATCATCCCGGGTTTCATGTGCCAGGGTGGTGACTTCACCCGTCACAACGGTACCGGTGGTAAATCTATCTACGGTGAAAAATTCGAAGACGAAAACTTCATCCTGAAACACACCGGTCCGGGTATCCTGTCTATGGCTAACGCTGGTCCGAACACCAACGGTTCTCAGTTCTTCATCTGCACCGCTAAAACCGAATGGCTGGACGGTAAACACGTTGTTTTCGGTAAAGTTAAAGAAGGTATGAACATCGTTGAAGCTATGGAACGTTTCGGTTCTCGTAACGGTAAAACCTCTAAAAAAATCACCATCGCTGACTGCGGTCAGCTGGAAGGTTACTGATCTCCTCGGATCC-3’
After verifying the correct sequence through DNA sequencing, the resulting plasmid with the fusion protein sequence (Supplemental Figure S1) was first amplified in TOP10 competent cells and then transfected into BLR competent cells for expression. After expression, the CA192 fusion protein was purified by inverse transition cycling (ITC) [27] (Table 1). Briefly, following cell lysis by sonication, the phase transition of CA192 can be triggered by heating to 37°C in the presence of 2 M sodium chloride (NaCl), allowing the collection of CA192 coacervates via centrifugation at 4,500 x g (hot spin). The ELP pellet was then resolubilized in cold PBS and subjected to another centrifugation at 4°C, a temperature below the Tt of CA192, at 16,100 x g (cold spin). This constituted one cycle of ITC. More than 98% purity was obtained by 3 rounds of ITC. The protein yield was around 90 mg/L to 120 mg/L.
Table 1.
Amino acid sequence and phase behavior of CA192.
| Label | Amino Acid Sequence | *M.W. [kDa] | **Slope, m [°C/Log10(μM)] | Intercept, b [°C] |
|---|---|---|---|---|
| A192 | G(VPGAG)192Y | 73.6 | 8.0 ± 1.7 | 71.8 ± 2.6 |
| CA192 | CypA-(VPGAG)192Y | 91.6 | 2.9 ± 2.2 | 53.1 ± 3.7 |
CypA amino acid sequence:
MVNPTVFFDIAVDGEPLGRVSFELFADKVPKTAENFRALSTGEKGFGYKGSCFHRIIPGFMCQGGDFTRHNGTGGKSIYGEKFEDENFILKHTGPGILSMANAGPNTNGSQFFICTAKTEWLKHVVFGKVKEGMNIVEAMERFGSRNGKTSKKITIADCGQLE
Expected molecular weight based on the open reading frame for the expressed protein.
The ELP transition temperatures were by Eq. (9), yielding an intercept, b, at 1 μM, and a slope, m, representing the change in temperature upon a 10-fold change in concentration. Mean ± 95% CI.
The concentration of CA192 in phosphate-buffered saline (PBS) was determined by measuring the optical density at 280 nm with a one-centimeter light path using a UV-Vis spectrophotometer (DU800, Beckman Coulter Inc., Brea, CA) after diluting CA192 into 6M guanidine hydrochloride to disrupt any aggregates. The molar extinction coefficient, ε, of CA192 and A192 were estimated to be 9,970 (M−1 cm−1) and 1,285 (M−1cm−1), respectively, based on the following equations [28]:
| Eq. 1 |
and
| Eq. 2 |
The molecular weight of purified fusion protein was verified by SDS-PAGE stained with copper chloride (CuCl2). The phase behavior of CA192, along with the parent ELP, A192, was characterized again using UV-Vis spectrophotometry by measuring the optical density at 350 nm, OD350, where neither fusion protein nor A192 contribute significantly to absorption. ELPs at different concentrations (5 μM to 100 μM) were subjected to a controlled temperature gradient from 25 to 75°C at 1°C/min. The transition temperature (Tt) of each ELP is defined as the temperature at which the first derivative of the optical density with respect to the temperature reaches a maximum.
2.4. Size exclusion chromatography (SEC) with multi-angle static light scattering (MALS)
To resolve CA192 and estimate the molecular weight of different populations of the protein, 100 μL of 25 μM of CA192 was injected onto a Shodex Protein KW-803 (8.0mm I.D. x 300 mm) (Showa Denko America, New York, NY) column preconditioned and equilibrated with PBS. This was followed by application of an isocratic flow of PBS at 0.5 mL/min was applied to elute CA192. Elution was monitored by three in-line detectors: i) UV 210 nm (SYS-LC-1200, Agilent Technologies, Santa Clara, CA); ii) multi-angle static light scattering (DAWN HELEOS, Wyatt Technology, Santa Barbara, CA); and iii) differential refractometer (OPTILAB rEX, Wyatt Technology, Santa Barbara, CA). ASTRA 6 was used for data analysis and molar mass determination.
2.5. Dynamic light scattering (DLS)
The hydrodynamic radius (Rh) of the ELP fusion proteins was measured via dynamic light scattering (DLS) using a DynaPro Plate Reader II from Wyatt Technology (Santa Barbara, CA) and analyzed by software DYNAMICS V7 (Wyatt Technology, Santa Barbara, CA). Before the DLS measurement, solutions were filtered through syringe filters (25 mm, 0.2 μm). The concentration of each solution was then adjusted to 20 μM. 60 μL from each sample was pipetted into three different wells on a 384 well clear bottomed plate and covered by 15 μL mineral oil in each well to avoid solvent evaporation. Centrifugation was performed to remove air bubbles prior to analysis.
2.6. Isothermal titration calorimetry (ITC)
ITC (MicroCal PEAQ-ITC, Malvern Instruments Ltd, Worcestershire, United Kingdom) was utilized to study the binding affinity between CsA and CA192. CsA and CA192 were solubilized in the same buffer (2.5% v/v DMSO in PBS) to eliminate the interference by background heat released from buffer disequilibrium. Then, the calorimeter cell was filled with 13 μM CsA and the titration syringe was filled with 150 μM CA192. The titration syringe injected 3 μL of CA192 12 times into the calorimeter cell. When binding occurs, the released heat during gradual titration is measured by the sensitive calorimeter. The MicroCal PEAQ ITC analysis software was then used to fit the resulting isotherm into an “one set of sites” binding model to generate the affinity (Kd), stoichiometry (N) and enthalpy of interaction (ΔH).
2.7. Drug encapsulation and reverse-phase high performance liquid chromatography (RP-HPLC) analysis
CsA was encapsulated into CA192 based on our previously reported two-phase solvent evaporation method [29]. Briefly, an aqueous phase (PBS containing 300 μM CA192) was mixed with an organic phase (90% hexane/10% ethanol containing 1 mM CsA). Under a nitrogen environment with constant stirring, along with the evaporation of organic solvent, CsA was gradually displaced into the aqueous phase where it was solubilized through binding CA192. This process was followed by high-speed centrifugation at 16,100 x g, ultrafiltration through syringe filters (25 mm, 0.2 μM) and dialysis for 4 hr using a dialysis cassette (20K MWCO, 3 mL) against PBS to remove excess insoluble drug and residual solvent. The loading ratio was determined by RP-HPLC using a C4 column (150 × 4.6 mm, particle size 5 μm, YMC CO., LTD.) The mobile phase was composed of water and methanol, each containing 0.1% trifluoroacetic acid (TFA). The sample was eluted at a flow rate of 1 ml/min with a gradient flow of methanol/water (40:60) to methanol/water (95:5) for the first 5 min and then an isocratic flow of methanol/water (95:5) for another 5 min. The eluate was subject to UV detection at 210 nm. CsA dissolved in methanol at different concentrations: 5 to 50 μM were first analyzed with this method to establish a standard curve.
2.8. In vitro drug release assay
The in vitro disassociation of CsA from CA192 (120 μM, 3 mL) in aqueous solution was characterized by performing sink dialysis against 1.5 L PBS at 4°C or 37°C. PBS was changed every 48 hr. Samples were collected from the dialysis cassette (20K MWCO, 3 mL) from 2 to 196 hr and analyzed by RP-HPLC. Similarly, to study the free drug release profile, CsA dissolved in DMSO was dialyzed against sink conditions of PBS at 4°C or 37°C, and sampled until 10 hr at 4°C or 5 hr at 37°C.
2.9. Competitive binding assays
To simulate the physiological situation where albumin may displace CsA from CA192, human serum albumin was dissolved into PBS solution with 150 μM CA192-CsA to a final concentration of 1 mM, which is approximately the physiological concentration of albumin in human serum. The mixture was incubated at 37 °C and sampled from 8 to 48 hr. The ELP-mediated phase-separation was then exploited to isolate CA192-CsA from the mixture. Briefly, 5M NaCl solution was added to collected samples to reach a final NaCl concentration of 1 M to induce the phase separation at 37°C. The pellet was obtained by centrifugation at 16,100 x g for 10 min and resuspended for RP-HPLC analysis to measure CsA concentration still retained by CA192.
CA192-CsA was also tested against mouse plasma collected from 26-week-old male BALB/c mice. 300 μM CA192-CsA in PBS was diluted 1:1 in mouse plasma to achieve a final concentration of 150 μM. Similarly, the mixture was incubated at 37 °C and sampled from 8 to 48 hr. The phase separation of CA192 was induced with 1M NaCl at 37 °C and the ELP was isolated by centrifugation. The pellet following centrifugation was resuspended for RP-HPLC analysis to measure the remaining CsA bound to CA192.
2.10. Cellular uptake of ELPs
CA192 was labeled with NHS-rhodamine. The labeling protocol was optimized for CA192 to achieve an approximately 100% labeling efficiency. Briefly, 3.5 mL of 200 μM CA192 was mixed with a 3X molar excess NHS-rhodamine at 4°C for 1.5 hr under constant rotation. Unbound, free dye was removed using Zeba Desalting Chromatography Cartridges (7K MWCO, 5 mL). The absorbance of rhodamine-CA192 (Rho-CA192) at 555 nm (OD555, RhoCA192) was measured using a UV-Vis spectrophotometer. The labeling efficiency was calculated based on the equation below, where the εrhodamine is 80,000 (M−1 cm−1) at 555 nm and the concentration of CCA192 was measured before labeling to be 200 μM.
| Eq. 3 |
Jurkat cells, Clone E6–1 (TIB-152™, ATCC®, Manassas, VA) were first cultured in medium composed of Advanced RPMI 1640 + 10% FBS + 10 mM HEPES + 100 units/mL penicillin + 100 μg/mL streptomycin to a density of 3 × 105/mL. Jurkat cells were then incubated on 35 mm glass bottomed dishes (P35G-0–10-C, MatTek Corporation, Ashland, MA) precoated with Poly-D-lysine for 15 min to enable attachment. Cell density was adjusted to 3 × 106/mL to achieve better adhesion and then diluted back to 3 × 105/mL for further treatment. Following attachment, cells were incubated with 10 μM rhodamine-labeled CA192 for 2 hr. Similarly, HeLa cells (CCL-2™, ATCC®, Manassas, VA) cultured to 50% confluency in Dulbecco’s Modified Eagle’s medium (DMEM) with L-glutamine with 10% Fetal Bovine Serum (FBS) and 100 units/mL penicillin + 100 μg/mL streptomycin were incubated with 30 μM rhodamine-labeled CA192 for 2hr. After washing three times with warm PBS to remove residual unbound Rho-CA192, cells were incubated with Hoechst (2 drops/mL culture media) and Lysotracker green at a final concentration of 150 nM for 20 min. Finally, cells were imaged by confocal fluorescence microscopy (ZEISS LSM 800 with Airyscan, Carl Zeiss Microscopy GmbH, Germany).
2.11. CsA-mediated suppression of IL-2 secretion from Jurkat cells
Jurkat cells, Clone E6–1 (TIB-152™, ATCC®, Manassas, VA) were cultured in medium composed of Advanced RPMI 1640 + 10% FBS + 10 mM HEPES + 100 units/mL penicillin + 100 μg/mL streptomycin to a density of 3×105/ml. Stimulation of Jurkat cells was with Phorbol 12-myristate 13-acetate (PMA) and ionomycin, each dissolved in DMSO and then added to culture medium to a final concentration of 20 ng/mL for PMA and 1 μg/mL for ionomycin. Immediately after the stimulation, cells were subject to treatment with CA192-CsA or free CsA dissolved in 2.5 % v/v DMSO at CsA concentrations from 10 pM to 100 nM for 6 hr at 37°C. IL-2 concentration in culture medium was assessed using an ELISA kit (EH2IL-2, Thermo Fisher Scientific, Waltham, MA) on a SpectraMax iD3 Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA) at 450 nm.
2.12. NFAT signaling using Western blot analysis
Jurkat cells were cultured as above in 6-well-plates to a cell density of 3×105/ml. Two wells were used as negative controls (non-treated) and positive controls (stimulation with PMA and ionomycin at doses listed above), respectively. The remainder of the wells were subjected to pre-incubation with CA192-CsA, CsA dissolved in 2.5% v/v DMSO, CA192, or 2.5 % v/v DMSO for 1 hr at 37 °C, followed by stimulation for another 1 hr at 37 °C. Cells were collected via centrifugation, washed three times with cold PBS, and lysed with RIPA lysis and extraction buffer containing Protease and Phosphatase Inhibitor Cocktail. Electrophoretic transfer was conducted with an iBlot 2 Dry Blotting System (Thermo Fisher Scientific, Waltham, MA) following electrophoretic separation using native PAGE or SDS-PAGE. After 1 hr blocking with 5% milk in Tris-buffered saline with 0.1% Tween 20 at room temperature, the membrane was incubated with primary rabbit antibody to NFAT1 (1:1000 dilution) or NFAT2 (1:1000 dilution) overnight at 4°C. After washing three times for 5 min, the membrane was incubated with anti-rabbit IgG, HRP-linked secondary antibody (1:1000 dilution) for 1 hr prior to washing three times for 5 min. Chemiluminescent substrate (ProSignal Dura components) was mixed 1:1 and placed on the blot with a volume of 0.1 mL/cm2 for 2 min, followed by draining of the excess reagent. The damp blot was imaged using a ChemiDoc Touch Imaging System (Bio-Rad Laboratories, Hercules, CA) to capture the chemiluminescent signal.
2.13. Pharmacokinetics and subcutaneous bioavailability of CA192
To study the pharmacokinetic profile of the CA192 carrier, Rho-CA192 (200 μM CA192 concentration; 104% labeling efficiency, 150 μL injection volume/35 g BW) was injected into 12-week male BALB/c mice intravenously (IV) or subcutaneously (SC) for a total dose of 30 nanomoles or 857 nanomoles / kg BW. 20 μL of blood was collected by tail nick at time points from 5 min to 72 hr. The collected blood was immediately added to 80 μL of heparinized PBS at a heparin concentration of 1000 U/ml. Red blood cells were removed by centrifugation at 16,100 x g for 10 min and diluted plasma was collected. CA192 concentration in plasma was calculated based on the fluorescence intensity measured by a SpectraMax iD3 Multi-Mode Microplate Reader (Excitation/Emission: 540/580 nm).
Both non-compartmental and compartmental methods were applied to analyze the PK profiles of CA192 after IV or SC administration. Non-compartmental analysis was primarily based on the estimation of body exposure to drug after administration, which is reflected by the area under the plasma concentration-time curve (AUC). The AUC was first computed with the trapezoidal method. Thereafter, the area under the first moment curve (AUMC), mean residence time (MRT) and mean absorption time (MAT) were calculated as follows:
| Eq. 4 |
| Eq. 5 |
Using these estimates, the SC bioavailability, F, the plasma clearance (CL) were estimated as follows:
| Eq. 6 |
| Eq. 7 |
Similarly, the terminal half-life, T1/2, Terminal, was best-fit to the log-linear decay observed in each individual over the last three time points.
Regarding the compartmental model-based analysis, the volume of distribution of the plasma compartment (Vd), the elimination rate constant (kelimination), the transfer rate constant from plasma to tissue (kplasma➜tissue) and the transfer rate constant from tissue back to plasma (ktissue➜plasma) were first solved from the two-compartmental IV model. This IV model was then used to construct the four-compartmental SC analysis under the assumption that Vd and kelimination remain constant from IV to SC. This enabled fitting of additional constants in the SC model including an apparent elimination rate constant from interstitial fluid (ISF) (kISF_elimination), which was used to account for the observed bioavailability, F. If either kISF_elimination or kSC_site->ISF were rate limiting (ie. much greater than the other), than the model would reduce to a one-phase absorption; however, a one-phase absorption model was unable to fit the late peak times observed. This suggested that kISF_elimination and kSC_site->ISF are on the same order of magnitude; therefore, the assumption was made that kISF_elimination = kSC_site->ISF, which enabled good fitting to each mouse. From these best-fit curves generated in SAAM II, the peak concentration, Cmax, and peak time, tmax, were extracted from calculated points of each fit after increasing the Minimum Number of Calculation Intervals to 500. For comparison with the noncompartmental CL, the clearance from the compartmental model was solved by the following equation:
| Eq. 8 |
2.14. Therapeutic evaluation of CA192/CsA using the male NOD mouse model of autoimmune dacryoadenitis in Sjögren’s Syndrome
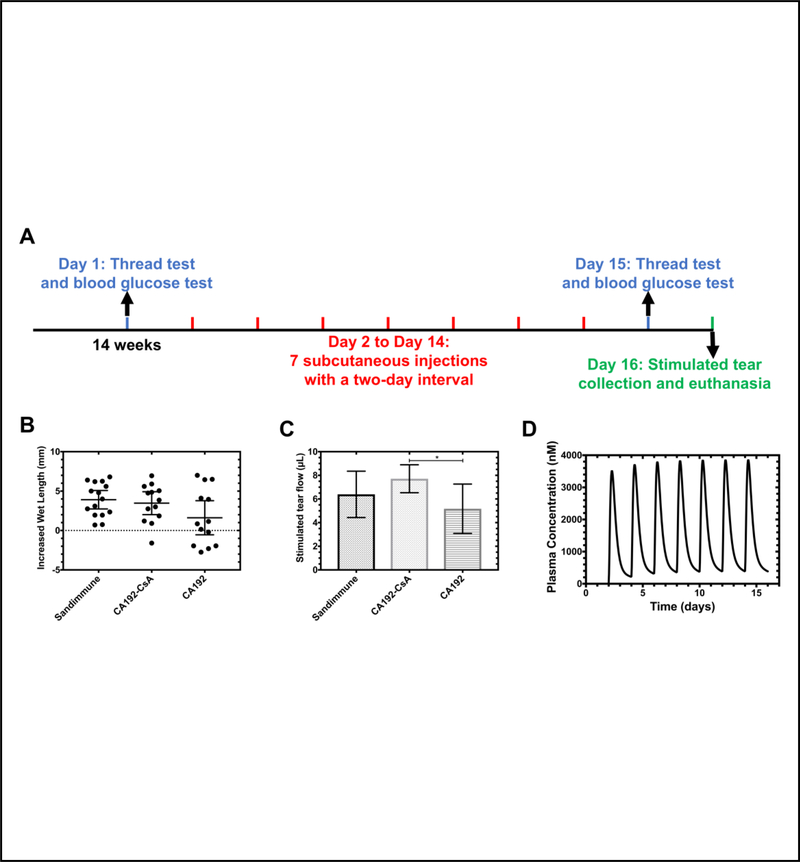
The therapeutic study on male NOD mice was initiated when animals reached 14 weeks of age, when SS-like autoimmune dacryoadenitis was fully established [30]. 45 mice were divided into 3 different groups receiving one of the three treatments: 1) CA192-CsA; 2) Free CsA (Sandimmune®); and 3) CA192 carrier control. For groups 1 and 3, 400 μL/35g BW of CA192 with a concentration of 300 μM with or without CsA loading, respectively, was injected. The same volume of diluted Sandimmune® was injected into mice in group 2. Mice in groups 1 and 2 were subject to the same CsA concentration of 2.5 mg/kg. Treatments were given SC every other day for 2 weeks. Basal tear production was measured before and after treatments by performing a thread test [31]. Briefly, under light anesthesia with isoflurane, a ZoneQuick phenol red-embedded thread was applied in both eyes at the canthus of the ocular surface for 10 sec. Basal tear volume was recorded as the length of thread wetting by basal tears in mm. Stimulated tear collection was conducted under full anesthesia as a terminal procedure. After intraperitoneal injection with a mixture of ketamine/xylazine at concentrations of 100 mg/kg and 10 mg/kg, respectively, mice were subjected to a small bilateral incision on the axis between the outer junction of the eyelid and the ear to expose the LG on both sides. Then 3 μL of 50 μM carbachol (CCh) was applied directly onto the LG to stimulate tear secretion, followed by tear collection from both eyes using 2 μL micro-capillary tubes, which were placed at the tear meniscus in the medial canthus for 5 min. This stimulation and collection procedure was repeated two more times and the volume of collected tears was recorded. Serum chemistry was conducted by ANTECH Diagnostics (Fountain Valley, CA).
3. Results
3.1. Protein purification and characterization
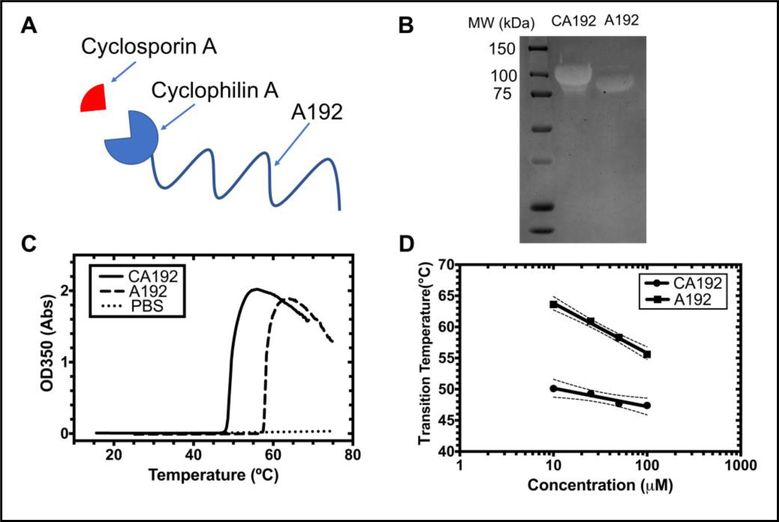
After expression, centrifugation and lysis, the CA192 fusion protein was concentrated and purified by repeatedly inducing ELP phase separation[27]. The molecular weight of purified fusion protein was verified by SDS-PAGE stained with copper chloride. The parent ELP, A192, served as a control. CypA has a molecular weight of 18.0 kDa. Combined with the 73.6 kDa molecular weight of A192, the molecular weight of CA192 was expected to be around 91.6kDa, which is consistent with the shift seen for CA192 on SDS-PAGE (Figure 1B).
Figure 1. A recombinant elastin-like polypeptide (ELP) fusion protein as a non-covalent drug carrier for cyclosporine A (CsA).
A) A gene was constructed encoding the cyclophilin A protein fused to a high molecular weight ELP, A192. This fusion, called CA192, binds specifically and with high affinity to CsA; B) SDS-PAGE of purified CA192 (91.6 kDa) stained with copper chloride demonstrates a molecular weight shift upon fusing CypA to A192 (73.6 kDa); C) Optical density was determined as a function of temperature (1°C/min) for 25 μM CA192, free A192, and a PBS control. The temperature with the maximum positive slope was defined as the phase transition temperature. D) The concentration-temperature phase diagrams of both CA192 and A192 are plotted, showing that both are expected to remain soluble at physiological temperatures. The 95% confidence interval around each best-fit line is indicated with dashed lines.
The phase transition temperature (Tt) of CA192 was reduced with respect to A192 (Figure 1C); however, both polymers likely remain soluble at physiological temperatures. At 25 μM, the transition temperature of CA192 was 49.3 °C, significantly lower than that of A192 at 60.9 °C. Consistent with our previous finding [32], the Tt of CA192 was also found to be a function of concentration:
| Eq. 9 |
where the intercept, b, representing the transition temperature at 1 μM, is equal to 53.1 °C, the slope, m, is the decrease in Celsius for a 10-fold increase in concentration, which equals 2.9 °C, and [CELP] represents the fusion protein concentration (Figure 1D, Table 1).
3.2. Particle size determination
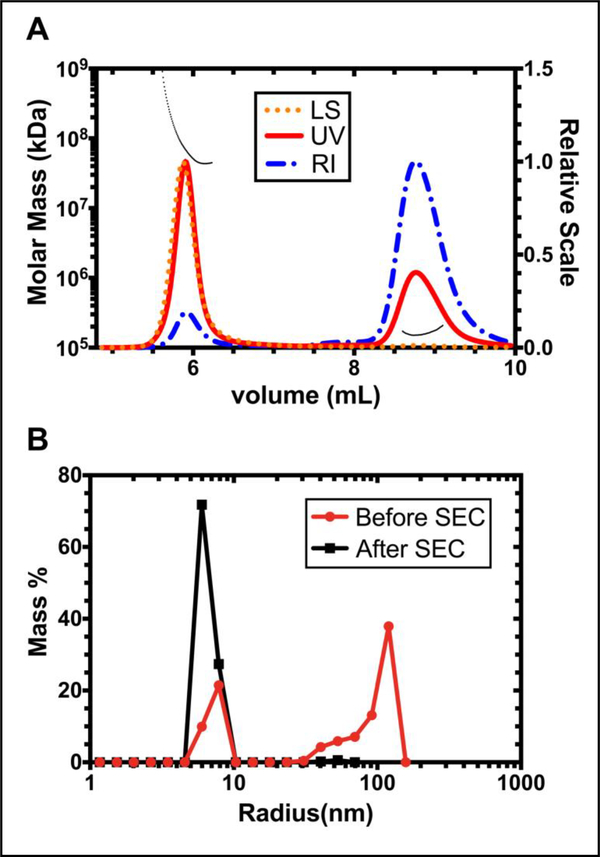
Size exclusion chromatography with multi-angle light scattering (SEC-MALS) was used to study the aggregation status and molecular weight of CA192. As shown in Figure 2A, two different fractions were separated by SEC, suggesting heterogeneity of CA192 in solution. The aggregation and oligomeric state of these two fractions was then evaluated by Multi-angle light scattering, together with a UV detector set at 210 nm and a differential refractometer. Surprisingly, the molecular weight of the second fraction was determined to be 181.0 kDa (± 4.1%), accurately doubling the expected molecular weight of CA192 in its monomeric state and indicating the existence of dimerized CA192. Similarly, fraction 1 was demonstrated to be an aggregated form based on its estimated molecular weight of 8.511 × 104 kDa (± 2.5%).
Figure 2. Expressed CA192 assembles two species in solution that can be isolated by size exclusion chromatography (SEC).
A) SEC-MALS was used to investigate the aggregation and oligomeric state of CA192. The molecular weight of these two fractions were determined to be 8.511 × 104 kDa (± 2.5%) and 181.0 kDa (± 4.1%), representing a nano-aggregate and a dimeric species, respectively. The black dashed line represents the molecular weight distribution. B) Purified CA192 dimers were isolated from the nano-aggregate population using SEC, which gave a monodisperse hydrodynamic radius of 6.9 ± 0.1 nm (mean ± SD, n=3).
Then the dimerized form was collected from SEC and subject to a DLS measurement of hydrodynamic radius (Rh). Native CA192 without SEC isolation was used as a control. As shown in Figure 2B, and consistent with data acquired from SEC-MALS, without further separation by SEC, DLS could detect two peaks in the native CA192 with Rh of 7.4 ± 0.7 nm (mean ± SD, n=3) and 113.0 ± 59.1 nm (mean ± SD, n=3). The isolated dimerized form, however, demonstrated a good monodispersity with a Rh of 6.9 ± 0.1 nm (mean ± SD, n=3).
3.3. Binding affinity of CA192 to CsA
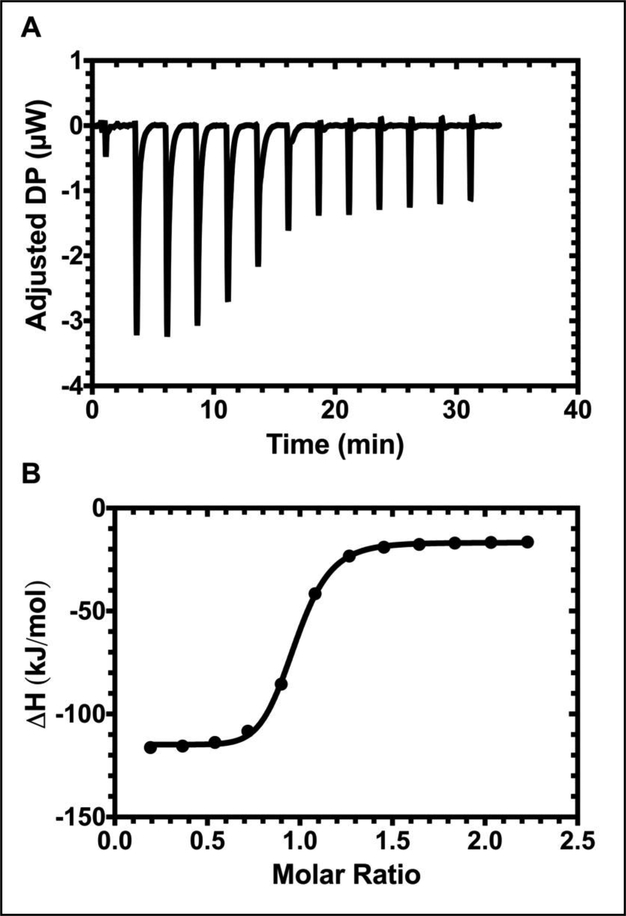
Isothermal titration calorimetry (ITC) was utilized to study the thermodynamics between CA192 and CsA. Two different fractions isolated by SEC were analyzed separately. Interestingly, we discovered that only dimerized CA192 maintains CsA binding capacity (Figure 3). Thus, from this point forward, only dimerized CA192 was used in our experiments. The dissociation constant (Kd) was determined to be 189 ± 87 nM (mean ± SD, n = 3) at 37 °C, which is slightly higher than that measured for endogenous CypA and CsA of 35.5 nM [33], possibly due to the fusion of CypA to the ELP construct and the presence of 2.5% DMSO in solution. Additionally, as expected, the stoichiometry indicated 1.05 ± 0.06 (mean ± SD, n = 3) consistent with the designed architecture, where one CA192 monomer binds to one CsA molecule.
Figure 3. Isothermal titration calorimetry confirms that dimeric CA192 maintains high binding affinity for CsA.
A) The heat pulse generated by serial injection of CA192 into a sample cell containing CsA ligand with respect to time. B) The titration curve generated by normalizing the released heat for concentration was fitted to a “one set of sites” binding model to generate the affinity (Kd), stoichiometry (N) and enthalpy of interaction (ΔH). They were determined to be 189.0 ± 87.4 nM, 1.05 ± 0.06 and −108.2 ± 16.8 kJ/mol, respectively.
3.4. In vitro drug release profile
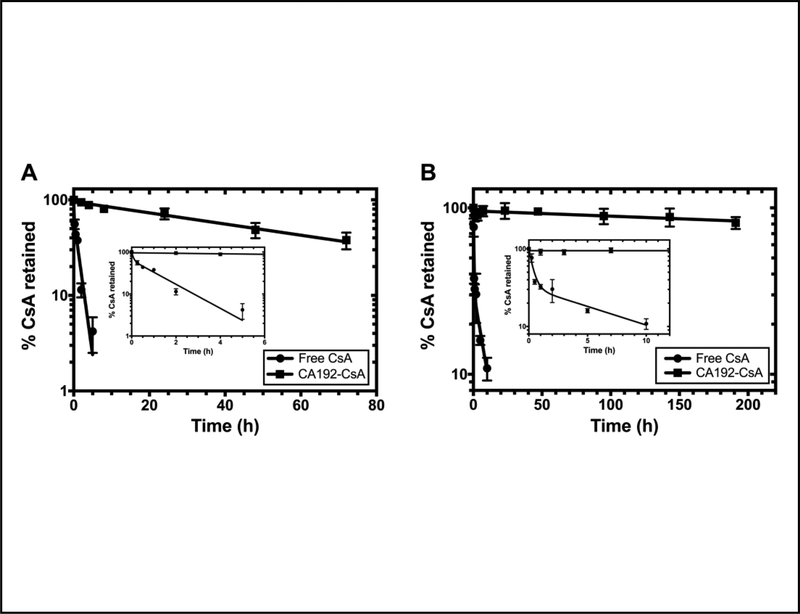
Binding to CA192 significantly altered the drug release profile of CsA in vitro as evaluated by dialysis under sink conditions at 4°C and 37°C. More than 75% of drug was still recovered in the dialysis cassette when stabilized by CA192 after 8 days at 4°C. As shown in Figure 4A and 4B, the drug release from CA192 fits a one-phase decay model with a half-life of 954 hr (95% CI: 553 to 3219 hr), of roughly 40 days, at 4°C and a half-life of 52 hr (95% CI: 44 to 61 hr) at 37°C. As a comparison, the free CsA release profile follows a two-phase decay with a burst release due to precipitation along with buffer exchange. The terminal half-life during the second slower decay is 6.3 hr (95% CI: 2.7 to 99.0 hr) at 4°C and 1.1 hr (95% CI: 0.8 to 1.6 hr) at 37°C.
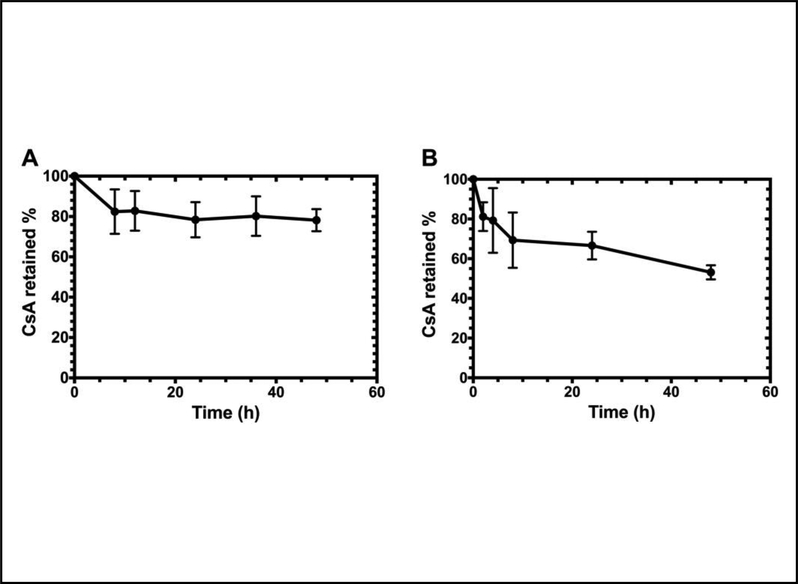
Figure 4. CA192 exhibits slow disassociation from CsA under sink dialysis in PBS.
In comparison with the rapid release of free CsA, CsA release from CA192 follows a one phase decay model with a half-life of A) 954 hr (95% CI: 553 to 3,219 hr) at 4°C or B) 52 hr (95% CI: 44 to 61 hr) at 37°C. Since loss of CsA loaded on CA192 is much slower than loss of Free CsA from the dialysis cassette, the disassociation kinetics from CA192 appear rate-limiting. Thus, the half-life for CA192-CsA reflects the kinetics of disassociation. Error bar represents mean ± SD from n=3.
3.4. Competitive binding assays
Albumin is the most abundant serum protein serving as a carrier for many hydrophobic molecules, which may potentially compete with CA192 to bind CsA during systemic circulation. Here we proposed an in vitro assay where 150 μM CA192-CsA was incubated with albumin at its physiological concentration, 1 mM. As shown in Figure 5, over a 48-hr period at 37°C, no significant drug loss from CA192 was observed, consistent with the high binding affinity reported in Figure 3. The initial drug loss during the first 8 hr can be explained by incomplete isolation of all of the CA192 from the mixture.
Figure 5. Neither physiological albumin nor whole plasma rapidly displaces CsA from CA192.
To simulate the physiological situation where purified albumin or lipoproteins may displace CA192 in binding CsA, 150 μM CA192-CsA was incubated with A) 1 mM albumin in PBS or B) heparinized mouse plasma for 2 days at 37°C. CA192 was isolated at different time points by ELP-mediated phase separation. Error bar represents mean ± SD from n=3.
Based on the manufacturer’s monograph on Sandimmune®, instead of albumin, lipoproteins in the plasma, mainly high-(HDL) and low-(LDL) density lipoprotein, predominantly bind to cyclosporine in the plasma. Thus, CA192-CsA was also tested against mouse plasma over a period of 48 hr. Despite an initial drug loss due to incomplete isolation during the hot spin, more than 50% of CsA was maintained as CA192 bound after 48 hr incubation at 37°C (Figure 5B). This degree of CsA loss is consistent with the 52 hr half-life of disassociation under sink dialysis at the same temperature (Figure 4B).
3.5. Cell uptake assay
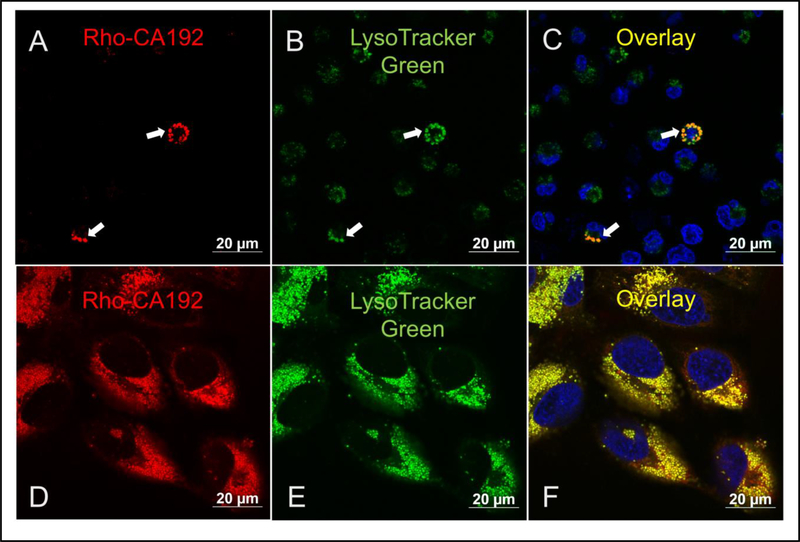
Jurkat cells were used to study whether CA192 can be internalized into cells as well to identify its intracellular distribution if internalized. Upon labeling with NHS-rhodamine, the internalization of CA192 was visualized by tracking the fluorescence signal emitted from rhodamine. As shown in Figure 6, notable fluorescence accumulation was detected in a punctate pattern observed in cells incubated with CA192 for 2 hr. The intracellular fluorescence was primarily co-localized with lysosomes labeled with Lysotracker Green, suggesting ultimate accumulation in lysosomes after uptake via some mode of endocytosis.
Figure 6. CA192 is internalized into Jurkat and Hela cells where it co-localizes with low pH compartments.
A-C) Jurkat Cells were incubated with Rho-CA192 (10 μM (Jurkat cells) or D-F) Hela cells (30 μM) for 2 hr. Unbound Rho-CA192 was then washed away three times with warmed PBS, and remaining A,D) Rho-CA192 was imaged (red) using confocal laser scanning microscopy. B,E) Lysosomes were stained with lysotracker green. C,F) Nuclei were labeled with Hoechst (blue) in an overlay. Bar represents 20 microns. Areas of strong colocalization is indicated by arrows.
3.6. In vitro efficacy
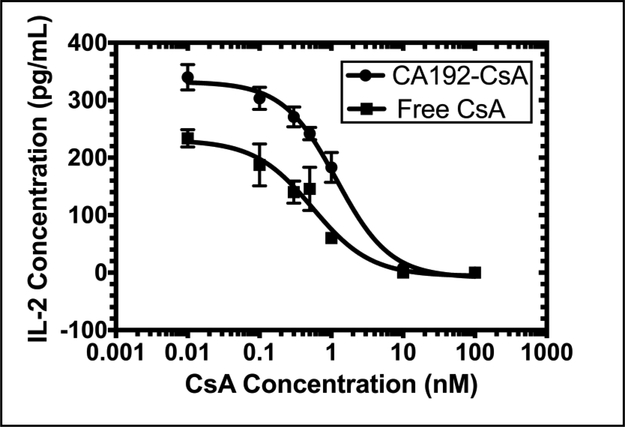
The in vitro efficacy of CA192-CsA was studied using the Jurkat cell line, an immortalized human T lymphocyte cell line which releases IL-2 in response to stimulation with PMA and ionomycin [34, 35], recapitulating T cell stimulatory responses in vivo. To determine whether the IL-2 release evoked by stimulation was neutralized effectively by CsA either in its free form or bound to CA192, stimulated cells were incubated with either CA192-CsA or free CsA dissolved in DMSO at serial dilutions indicated for 6 hr. The respective inhibitory effects were quantified through measurement of released IL-2 concentration into the cell culture medium using an ELISA. As shown in Figure 7, both CA192-CsA and CsA/DMSO evoked substantial inhibition of IL-2 secretion at sub-nanomolar concentrations. The half maximal inhibitory concentration (IC50) of CA192-CsA was 1.2 ± 0.4 nM (n = 3, mean ± SD), slightly higher than that of CsA in DMSO of 0.5 ± 0.2 nM (n = 3, mean ± SD).
Figure 7. CA192-CsA exhibits comparable IL-2 inhibition efficacy to free CsA.
Upon stimulation, Jurkat cell produce large amounts of IL-2. CsA can arrest this process by inhibiting calcineurin and the NFAT signaling pathway. The IC50 of CA192-CsA was determined to be 1.2 ± 0.4 nM (mean ± SD from n = 3), slightly higher than free CsA with an IC50 of 0.5 ± 0.2 nM (mean ± SD from n = 3).
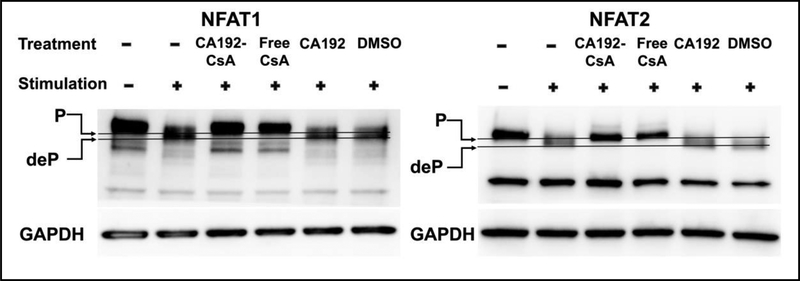
To confirm whether this inhibitory effect was achieved through conventional calcineurin and NFAT signaling, we evaluated the effects of CA192-CsA versus free CsA on the phosphorylation state of NFAT1 and NFAT2 in activated Jurkat cells. As shown in Figure 8, CA192-CsA, as well as the CsA positive control in DMSO were able to arrest NFATs in their inactive phosphorylated state. The negative controls, CA192 or DMSO, did not reverse the dephosphorylation of NFATs by calcineurin evoked by PMA and ionomycin. The phosphorylated NFATs were differentiated from the dephosphorylated NFATs based on their migration on SDS-PAGE.
Figure 8. CA192-CsA prevents NFAT1 and NFAT2 dephosphorylation.
10 μg of total protein in the supernatant of cell lysate was resolve in each lane by SDS-PAGE. Proteins on gels were transferred to membrane and labeled with anti-NFAT1 or 2 primary rabbit antibodies followed by anti-rabbit IgG, HRP-linked secondary antibody. Jurkat cell pretreatments were with CA192-CsA or with free CsA (dissolved in 2.5% v/v DMSO) at 1 μM CsA concentration, or with vehicle controls, prior to stimulation with 20 ng/mL PMA and 1 μg/mL ionomycin. CA192-CsA and free CsA treatments showed retention of NFAT1 and 2 at their phosphorylated forms with stimulation but neither CA192 vehicle nor DMSO control (2.5%v/v) prevented NFAT dephosphorylation upon stimulation. The cell lysis method used here was not optimized for nuclear protein extraction, which explains why the bands of dephosphorylated NFATs have lower intensity associated with nuclear translocation. The blots shown here are representatives of three independent repeats.
3.7. Pharmacokinetic profile of CA192
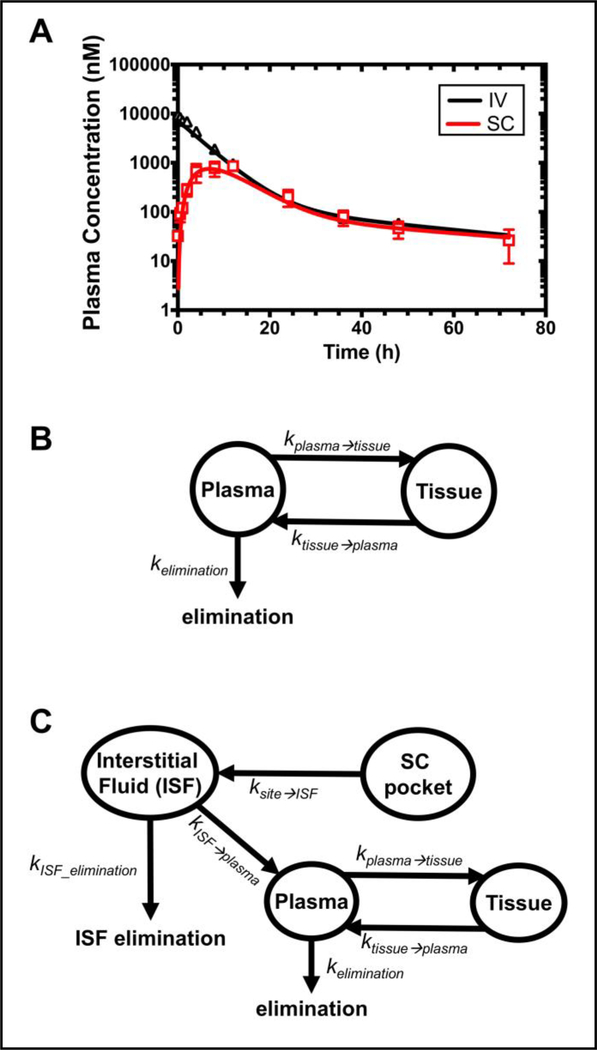
The PK profile of CA192 administered intravenously or subcutaneously was investigated in 12-week male BALB/c mice (28.3 ± 1.4 g BW, n=10). The plasma concentration of CA192 was converted from retained fluorescence intensity in the plasma. The plasma concentration of CA192 versus time profile is depicted in Figure 9A. Then both non-compartmental and compartmental analyses were utilized to characterize and interpret the PK profile of CA192, with each mouse analyzed individually to obtain statistical reliability. The estimated PK parameters of CA192 are summarized in Table 2. Based on non-compartment analysis, the MRT was extended from 7.3 hr to 15.9 hr through switching IV to SC administration, which reflects a mean absorption time (MAT) of 8.6 hr. The bioavailability, F, of SC administration was determined to be 30.9%. The terminal half-life observed was of 29.2, 22.4 hr for IV, SC administration respectively. This long terminal half-life results from the strong biphasic elimination observed as well as the high MW (182 kDa) of dimeric CA192.
Figure 9. Pharmacokinetic profile of CA192 dosed via IV or SC.
A) Plasma concentration of CA192 over time following IV or SC administration. Error bars represent mean ± SD from n = 5; B) The compartmental analysis of CA192 administrated via IV injection. A tissue compartment was suggested by its two-phase exponential decay profile; C) The compartmental analysis of CA192 administered via SC injection. This model could be consistent with absorption first into interstitial fluid (ISF) and from there was drained into blood circulation.
Table 2.
Comparison of pharmacokinetic parameters observed for dimeric CA192 administered by IV and SC administration to mice
| Parameter (Unit) | Route of Administration | |
|---|---|---|
| IV, (n=5) | SC, (n=5) | |
| CL/F (mL/hr) | 0.49 (0.05) | 1.66 (0.36) |
| AUC (μM hr) | 50.7 (3.8) | 15.7 (3.7) |
| AUMC ( μM hr 2) | 371.9 (50.4) | 248.9 (60.7) |
| MRT (hr) | 7.3 (0.8) | 15.9 (1.2) |
| MAT (hr) | - | 8.6 (1.4) |
| F (%) | 100 | 30.9 (7.3) |
| T1/2, Terminal (hr) | 29.2 (15.2) | 22.4 (11.7) |
| Model used | Two Compartment | Four Compartment |
| CL (mL/hr) | 0.51 (0.07) | *0.51 |
| Vd (mL/g BW) | 0.112 (0.013) | *0.112 |
| Cmax ( μM) | 8.6 (1.6) | 0.8 (0.3) |
| tmax (hr) | 0 | 7.1 (1.1) |
| kelimination (hr−1) | 0.16 (0.03) | *0.16 |
| ktissue➜plasma (hr−1) | 0.023 (0.019) | 0.025 (0.011) |
| kplasma➜tissue (hr−1) | 0.024 (0.003) | 0.072 (0.058) |
| ksite➜ISF (hr−1) | - | **0.19 (0.02) |
| kISF➜plasma (hr−1) | - | |
| kISF_elimination (hr−1) | - | 0.43 (0.18) |
AUC, AUMC, MRT, MAT, F, and T1/2, Terminal were calculated using non-compartmental analysis; The remaining parameters are based on compartmental models optimized to fit the data, as shown in Figure 9B and 9C. Values are indicated as the mean (SD).
Compartmental model parameters obtained from the IV analysis were fixed in the SC analysis.
To avoid over-parameterizing the four-compartment SC model both first order absorption rate constants were assumed to equal.
To better understand the PK for CA192, compartmental models were next developed that fit the observed data, as shown Figure 9B, 9C. IV CA192 followed a biexponential decay, which was interpreted using a two-compartment pharmacokinetic model. This model assumes the initial distribution of CA192 into an apparent volume of distribution, Vd, from which it distributed to surrounding tissues slowly during eventual elimination from the central plasma compartment. The best estimates for Vd and kelimination from the intravenous two-compartment model were the input into a model for SC administration. When a three-compartment SC model was evaluated for direct absorption from the injection site to the plasma, it was unable to fit the late peak times observed. To accommodate this delay, a four-compartment model was required with a two-phase absorption phase from the subcutaneous injection site to another pool, which may represent the ISF. This second ‘ISF’ pool then drives absorption into the central plasma compartment. In addition, direct elimination from the ‘ISF’ was allowed to account for the observed bioavailability of ~30%, which may reflect degradation of the Rh-CA192 in ISF or elsewhere en route back to the circulatory system. To fit the profiles for each mouse reliably, it was necessary to assume that the absorption rate constants from the SC site to the ISF and from the ISF to the plasma were equal, which reflects that they are on the same order of magnitude. Both of these models are able to accurately fit the observed profiles (Figure. 9), and their fit parameters are summarized (Table 2). The compartmental model also enables the determination of the peak concentration after SC administration, Cmax = 0.8 μM, which occurred at tmax = 7.1 hrs. Moreover, the clearances derived from noncompartmental, compartmental IV administration of CA192 were 0.49, 0.51 mL/hr respectively, which are in close agreement.
3.8. Therapeutic effect on the NOD mouse model
2.5 mg/kg of CsA delivered by CA192 was administered SC following the regimen depicted in Figure 10A to male NOD mice with established disease aged 14 weeks. Sandimmune (IV), introduced as a free drug positive control, was injected SC at the same dose. CA192 without CsA was used as a negative vehicle control. Since NOD mice have a tendency to develop type 1 diabetes with onset between 4–6 months of age, blood glucose was monitored during the study. As shown in Supplemental Figure S2, throughout the treatment period, non-fasting blood glucose of every mouse remained below the 250 mg/dL threshold, taken as the onset of diabetes, suggesting that they remained diabetes-free for the duration of the study. Basal tear flow before and after experimental treatments were measured by the thread test and compared. If the basal tear production increased after the treatment, it was interpreted that the mouse benefited from the treatment. Without CsA, the general trend is towards decreased tear production due to progression of inflammation during the study. As shown in Figure 10D, most mice treated with Sandimmune or CA192-CsA benefited from treatment, while approximately half of the mice in the control group with CA192 alone showed no benefit. In addition to basal tear production, carbachol-stimulated tear production was compared among groups. Results in Figure 10C clearly indicate that Sandimmune and CA192-CsA treated groups exhibited higher stimulated tear production relative to the CA192-treated control group. Notably, CA192-CsA treated mice showed a significantly higher tear production than CA192-treated mice. CA192-CsA did not significantly increase stimulated tear production relative to Sandimmune, which was likely because Sandimmune showed a trend to an increased production that did not rise to statistical significance. To provide insight into the expected plasma levels for CA192 during this study, the optimal compartmental model for SC CA192 (Table 2), was then used to predict the plasma concentrations expected during this entire course of treatment (Figure 10D). In addition to predicting the CA192 levels at assay days 15 and 16, this prediction shows that a low level of accumulation is expected during the first four days of therapy, after which levels of the carrier follow a steady state profile.
Figure 10. Subcutaneous administration of CA192-CsA increased basal and stimulated tear production in a mouse model of SS.
A) The schedule of dosing and acquisition of blood glucose and tear production measurements; B) Basal tear production measure by phenol-red threads suggested that most mice treated with Sandimmune or CA192-CsA benefited from the treatment: only the mean ± 95% CI of CA192 group includes 0; C) On the day of euthanasia, tears were collected after stimulating the LG topically with carbachol as described in the methods. CA192-CsA treated mice exhibited a significant increase in tear volume relative to CA192 control (P = 0.014). Error bars here represent mean ± SD from n=15; D) Using the compartmental model for SC administration (Table 2), the expected concentration profile for CA192 over the duration of the two-week study was estimated.
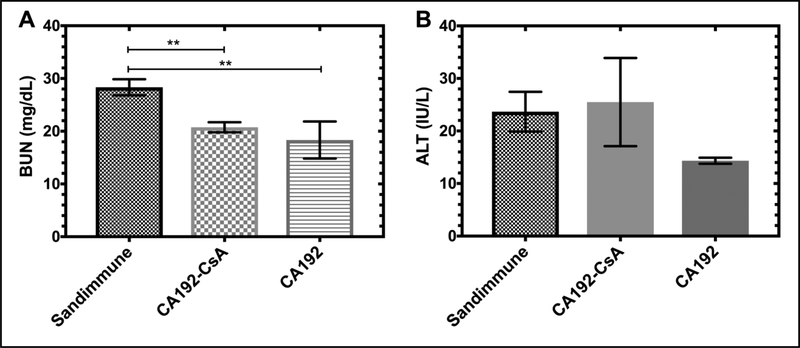
The most common clinical side effects of CsA, when administered systemically, are nephrotoxicity and hepatotoxicity[36]. Thus, we used blood urea nitrogen (BUN) as a nephrotoxicity biomarker to assess renal function. Interestingly, serum chemistry demonstrated that BUN in the Sandimmune group was approximately 50% higher than in the CA192-CsA and CA192 groups (Figure 11A). Similarly, serum alanine transaminase (ALT) was used to assess hepatotoxicity. However, no significant difference among groups was observed (Figure 11B).
Figure 11. CA192 delivery suppressed CsA nephrotoxicity relative to free CsA.
A) Blood urea nitrogen (BUN) is a biomarker of nephrotoxicity. Serum chemistry demonstrated that Sandimmune treated mice had significantly higher BUN than CA192-CsA or CA192 treated mice, indicating higher nephrotoxicity. B) Alanine aminotransferase (ALT), an indicator for hepatotoxicity, was also inspected but no differences in values were observed between groups. Three animals from each group were subject to serum chemistry testing. Error bars represent mean ± SD from n = 3.
4. Discussion
SS is a common chronic autoimmune disorder affecting more than 4 million Americans. Persistent DES is one of the clinical hallmarks of SS and can eventually lead to severe corneal damage. One of the few prescribed treatments available for SS-associate DES is an ophthalmic emulsion containing 0.05% CsA (RESTASIS®), which is intended to increase tear production as well as manage ocular surface inflammation. However, RESTASIS® showed disappointingly inadequate clinical efficacy in inflammation-related DES in clinical trials where only 15% of RESTASIS® treated patients exhibited statistically significant increases in a Schirmer’s wetting test. More importantly, it did not restore LG tear production in SS patients [37–39]. For treatment of DES originating with an aqueous tear production deficiency like SS that is principally due to LG deficiency, this is a critical limitation. This inability of CsA to affect the LG may be because the nasolacrimal ducts efficiently drain topically-applied drugs from the ocular surface, allowing only limited systemic absorption at the LG and other sites of inflammation in SS. Topical administration may thus similarly limit other new treatments intended for dry eye, such as the LFA-1 antagonist, lifitegrast (Xiidra™) and emerging techniques, including nanowafers or microgels, from being fully effective in SS-associated dry eye [40, 41]. In contrast, the new CsA delivery platform reported here clearly has the ability to restore stimulated tear production from the LG in a mouse model of SS, which topical CsA was unable to clinically achieve. While future studies will investigate in more detail the additional potential therapeutic systemic benefits that this new SC CsA formulation has on exocrine gland inflammation and development of serum autoantibodies, as well as its effects on systemic inflammatory pathways, our proof-of-principle study shows that CA192 shows significant promise in mitigating the efficacy issues associated with use of CsA in SS-associated DES. It does so while reducing dose-limiting toxicities that have been associated with CsA administration for other autoimmune and transplant conditions.
As discussed above, topical administration of CsA, despite being noninvasive and accessible, has limited systemic absorption which limits its ability to address many of the underlying mechanisms of SS and other autoimmune diseases. Therefore, several liposome or phospholipid micelle-based CsA carriers for systemic administration have been investigated as replacements for Cremophor EL® to overcome the poor water solubility and dose-limiting systemic toxicity of CsA, where reduced nephrotoxicity and improved drug disposition in vivo were observed [42, 43]. However, recognized as foreign substances, liposomes or phospholipid micelles encounter multiple defense systems including reticuloendothelial system (RES), opsonization and immunogenicity [44]. We report here our novel protein-based CsA carrier, CA192, which has the following advantageous properties. First, distinct from conventional liposomes or phospholipid micelles, which are known to be both effective and mildly immunogenic [45, 46], CA192 may be immunologically acceptable and fully biodegradable as both moieties of this fusion protein are initially derived from human self proteins. Second, the hydrodynamic radius of dimeric CA192 was adjusted to approximately 7 nm to allow a favorable SC absorption and biodistribution profile with a particle size exceeding the renal filtration cutoff to permit extended circulation. Third, the high binding affinity of CA192 for CsA (Figure. 3) greatly enhances drug solubility and provides a very long duration, one-phase drug release with minimal burst release (Figures. 4, 5), an improvement not possible with the current liposomal or micelle-based CsA carriers. Finally, since we have shown that CsA can be well maintained in its CA192-bound state without being sequestered by albumin or lipoproteins in the plasma, less drug-drug interaction may be expected when co-administered with other drugs that intensively bind to albumin or lipoproteins, such as methotrexate [47, 48] or docetaxel [49, 50], respectively. This strategy may make possible modification with targeting peptides to further improve the enrichment in inflamed tissue while reducing the off-target toxicity of CsA.
Regarding the feasibility of advancement from the laboratory scale to a clinical setting, the critical problem we will need to address in the future is to increase the drug loading capacity from the current 0.6% by mass to approximately 5% by mass, comparable to most drug delivery platforms. Although the high yield and ease of purification of ELP-based recombinant fusion proteins makes it possible to scale up from an animal study to clinical studies, where a substantially higher dose is required, subcutaneous injection may need to be replaced by intravenous or intraperitoneal injection. These routes of infusion permit safe administration of higher volumes; however, the high solubility and low burst release of this formulation may also promote local administration, such as intra-lacrimal gland or subconjunctival injection. When CA192 at a higher loading capacity is administered to humans, additional toxicity of CsA may emerge. Our current data demonstrates the efficacy of CA192 to curtail nephrotoxicity, which may extend to related CypA-ELP fusions with higher loading capacity, receptor-mediated targeting ligands, or phase separation at physiological temperatures.
Not only being advantageous over traditional drug carriers, the concept of utilizing the cognate receptor small molecule drug conjugated to ELPs through molecular cloning as a drug carrier, reported herein and previously by our lab [15, 51, 52], is potentially superior to other ELP-based small molecule carriers as well. Functional CA192 fusion protein can be directly synthesized and easily purified by exploiting the ELP phase behavior from E. coli with a high yield of 90 −120 mg/L, while other ELP-mediated delivery of small molecules require chemical conjugation for drug attachment [53–55], introducing chemical variability and polydispersity, thus, necessitating further chromatographic purification.
Another question that we will address in future studies utilizing this carrier is the role of of IL-2 in SS. On one hand, intralesional T cells were demonstrated to predominantly express Th1 cytokines including IL-2 and IFN-γ in SS patients [56, 57]. Over 40-fold more IL-2 is demonstrated to be produced by CD4+ T cells in SG of SS patients as well [23]. Thus, an inhibitor effect of CsA on IL-2 should have specific therapeutic potential in the treatment of T cell exocrine gland infiltration in SS. On the other hand, emerging studies have begun to establish a protective role of IL-2 in some autoimmune diseases [58] due to the key role IL-2 plays in the homeostasis and activation of regulatory T cells (Tregs) [59]. However, since Tregs are 7 to 10 times more sensitive to IL-2 than natural killer (NK) cells and other IL-2 responsive CD4+ helper T cells [60], IL-2 levels needs to be constrained to an “ultra-low-dose” range where Tregs can still reliably expand but which has relatively less impact on other IL-2–responsive cells. Therefore, identifying an optimal CsA dose for the specific autoimmune disease of interest, whether it is SS or another, will be critical in future studies. In addition to IL-2 downregulation, the therapeutic efficacy of CsA on SS may be highlighted through its potential inhibitory effects on IL-17 as well [61, 62], whose contribution in immunopathogenesis in various autoimmune disorders, including SS [63–66], has been newly suggested by mounting evidence.
5. Conclusions
Here we demonstrate for the first time that the prolyl isomerase protein known as CypA, can be bioengineered into a drug carrier, CA192, for the potent immunosuppressant CsA. Unlike traditional drug encapsulation strategies, this innovative strategy is surfactant-free, does not require the breakage of a covalent linkage, and is instead based on high specificity binding between a drug and its cognate receptor protein. Since the MW of CypA is below the renal filtration cutoff, it was fused to a humanized elastin-like polypeptide to increase its MW by ~80 kDa; furthermore, this fusion assembles a stable, functional, and dimeric species. When bound to CsA this carrier retains drug for extended durations, traffics to low pH compartments in cells, inhibits the NFAT/Calcineurin/IL-2 pathway, enhances the mean residence time following subcutaneous administration, reduces renal drug toxicity, and increases tear production in a non-obese diabetic mouse model of SS.
Supplementary Material
Acknowledgements
The authors would like to thank Francie Yarber for her contribution in the animal studies. This work was made possible by the University of Southern California (USC), the National Institute of Health R01 EY026635 to JAM and SHM, RO1 GM114839 to JAM, P30 EY029220 to the USC Ophthalmology Center Core Grant for Vision Research, P30 CA014089 to the USC Norris Comprehensive Cancer Center, P30 DK048522 to the Liver Histology Core of the USC Research Center for Liver Diseases, the Gavin S. Herbert Endowed Chair of Pharmaceutical Sciences, the L.K. Whittier Foundation, the USC Nano Biophysics Core Facility, and the Translational Research Laboratory at USC School of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Stevenson W, Chauhan SK, Dana R, Dry eye disease: an immune-mediated ocular surface disorder, Archives of Ophthalmology, 130 (2012) 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gijtenbeek J, Van den Bent M, Vecht CJ, Cyclosporine neurotoxicity: a review, Journal of neurology, 246 (1999) 339–346. [DOI] [PubMed] [Google Scholar]
- [3].Shaw J-P, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR, Idetification of a Putative Regulator of Early T Cell Activation Genes, Science, 241 (1988) 202. [DOI] [PubMed] [Google Scholar]
- [4].Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopiński P, Immunomodulation on the ocular surface: a review, Central-European journal of immunology, 41 (2016) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Colombo D, Ammirati E, Cyclosporine in transplantation—a history of converging timelines, Journal of biological regulators and homeostatic agents, 25 (2011) 493. [PubMed] [Google Scholar]
- [6].Mahalati K, Belitsky P, West K, Kiberd B, Fraser A, Sketris I, Macdonald AS, McAlister V, Lawen J, Approaching the therapeutic window for cyclosporine in kidney transplantation: a prospective study, Journal of the American Society of Nephrology, 12 (2001) 828–833. [DOI] [PubMed] [Google Scholar]
- [7].Bennett WM, Pulliam JP, Cyclosporine nephrotoxicity, Annals of internal medicine, 99 (1983) 851–854. [DOI] [PubMed] [Google Scholar]
- [8].Kassianides C, Nussenblatt R, Palestine AG, Mellow SD, Hoofnagle JH, Liver injury from cyclosporine A, Digestive diseases and sciences, 35 (1990) 693–697. [DOI] [PubMed] [Google Scholar]
- [9].Bellet M, Cabrol C, Sassano P, Léger P, Corvol P, Ménard J, Systemic hypertension after cardiac transplantation: effect of cyclosporine on the renin-angiotensin-aldosterone system, The American journal of cardiology, 56 (1985) 927–931. [DOI] [PubMed] [Google Scholar]
- [10].Cornec D, Saraux A, Jousse-Joulin S, Pers J-O, Boisramé-Gastrin S, Renaudineau Y, Gauvin Y, Roguedas-Contios A-M, Genestet S, Chastaing M, The differential diagnosis of dry eyes, dry mouth, and parotidomegaly: a comprehensive review, Clinical reviews in allergy & immunology, 49 (2015) 278–287. [DOI] [PubMed] [Google Scholar]
- [11].Janine A, The epidemiology of dry eye disease: report of the epidemiological subcommittee of the international dry eye workshop, Ocul Surf, 5 (2007) 93–107. [DOI] [PubMed] [Google Scholar]
- [12].Gupta C, Chauhan A, Ophthalmic delivery of cyclosporine A by punctal plugs, J Control Release, 150 (2011) 70–76. [DOI] [PubMed] [Google Scholar]
- [13].Lemp MA, Dry eye (keratoconjunctivitis sicca), rheumatoid arthritis, and Sjögren’s syndrome, in, Elsevier, 2005. [DOI] [PubMed] [Google Scholar]
- [14].Manoussakis MN, Moutsopoulos HM, Sjögren’s syndrome: autoimmune epithelitis, Best Practice & Research Clinical Rheumatology, 14 (2000) 73–95. [DOI] [PubMed] [Google Scholar]
- [15].Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, Louie SG, Rodgers K, MacKay JA, Hamm-Alvarez SF, A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjögren’s syndrome, Journal of controlled release, 171 (2013) 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].da Costa SR, Wu K, Veigh M. Mac, Pidgeon M, Ding C, Schechter JE, Hamm-Alvarez SF, Male NOD mouse external lacrimal glands exhibit profound changes in the exocytotic pathway early in postnatal development, Experimental eye research, 82 (2006) 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chiorini J, Cihakova D, Ouellette C, Caturegli P, Sjögren syndrome: advances in the pathogenesis from animal models, Journal of autoimmunity, 33 (2009) 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schenke-Layland K, Xie J, Angelis E, Starcher B, Wu K, Riemann I, MacLellan WR, Hamm-Alvarez SF, Increased degradation of extracellular matrix structures of lacrimal glands implicated in the pathogenesis of Sjögren’s syndrome, Matrix Biology, 27 (2008) 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yamano S, Atkinson JC, Baum BJ, Fox PC, Salivary gland cytokine expression in NOD and normal BALB/c mice, Clinical Immunology, 92 (1999) 265–275. [DOI] [PubMed] [Google Scholar]
- [20].Robinson CP, Cornelius J, Bounous DI, Yamamoto H, Humphreys-Beher MG, Peck AB, Infiltrating lymphocyte populations and cytokine production in the salivary and lacrimal glands of autoimmune NOD mice, Lacrimal gland, tear film, and dry eyes syndromes, 2 (1998) 493–497. [DOI] [PubMed] [Google Scholar]
- [21].Lindqvist AK, Nakken B, Sundler M, Kjellen P, Jonsson R, Holmdahl R, Skarstein K, Influence on spontaneous tissue inflammation by the major histocompatibility complex region in the nonobese diabetic mouse, Scandinavian journal of immunology, 61 (2005) 119–127. [DOI] [PubMed] [Google Scholar]
- [22].Aluri HS, Kublin CL, Thotakura S, Armaos H, Samizadeh M, Hawley D, Thomas WM, Leavis P, Makarenkova HP, Zoukhri D, Role of matrix metalloproteinases 2 and 9 in lacrimal gland disease in animal models of Sjögren’s syndrome, Investigative ophthalmology & visual science, 56 (2015) 5218–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fox RI, Kang H-I, Ando D, Abrams J, Pisa E, Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome, The Journal of Immunology, 152 (1994) 5532–5539. [PubMed] [Google Scholar]
- [24].Dhandhukia J, Weitzhandler I, Wang W, MacKay JA, Switchable elastin-like polypeptides that respond to chemical inducers of dimerization, Biomacromolecules, 14 (2013) 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Urry DW, Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers, The Journal of Physical Chemistry B, 101 (1997) 11007–11028. [Google Scholar]
- [26].Janib SM, Pastuszka M, Aluri S, Folchman-Wagner Z, Hsueh PY, Shi P, Yi A, Cui H, Mackay JA, A quantitative recipe for engineering protein polymer nanoparticles, Polym. Chem, 5 (2014) 1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sun G, Hsueh P-Y, Janib SM, Hamm-Alvarez S, MacKay JA, Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain, Journal of controlled release, 155 (2011) 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gill SC, Von Hippel PH, Calculation of protein extinction coefficients from amino acid sequence data, Analytical biochemistry, 182 (1989) 319–326. [DOI] [PubMed] [Google Scholar]
- [29].Shi P, Aluri S, Lin YA, Shah M, Edman M, Dhandhukia J, Cui H, MacKay JA, Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo, J Control Release, 171 (2013) 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hunger RE, Carnaud C, Vogt I, Mueller C, Male gonadal environment paradoxically promotes dacryoadenitis in nonobese diabetic mice, The Journal of clinical investigation, 101 (1998) 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shah M, Edman MC, Janga SR, Yarber F, Meng Z, Klinngam W, Bushman J, Ma T, Liu S, Louie S, Rapamycin eye drops suppress lacrimal gland inflammation in a murine model of Sjögren’s syndrome, Investigative ophthalmology & visual science, 58 (2017) 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang W, Jashnani A, Aluri SR, Gustafson JA, Hsueh P-Y, Yarber F, McKown RL, Laurie GW, Hamm-Alvarez SF, MacKay JA, A thermo-responsive protein treatment for dry eyes, Journal of Controlled Release, 199 (2015) 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wear MA, Walkinshaw MD, Thermodynamics of the cyclophilin-A/cyclosporin-A interaction: a direct comparison of parameters determined by surface plasmon resonance using Biacore T100 and isothermal titration calorimetry, Analytical biochemistry, 359 (2006) 285–287. [DOI] [PubMed] [Google Scholar]
- [34].Zhu P, Jiang W, Cao L, Yu W, Pei Y, Yang X, Wan B, Liu JO, Yi Q, Yu L, IL-2 mRNA stabilization upon PMA stimulation is dependent on NF90-Ser647 phosphorylation by protein kinase CβI, The Journal of Immunology, 185 (2010) 5140–5149. [DOI] [PubMed] [Google Scholar]
- [35].Andersson J, Nagy S, Groth C, Andersson U, Effects of FK506 and cyclosporin A on cytokine production studied in vitro at a single-cell level, Immunology, 75 (1992) 136. [PMC free article] [PubMed] [Google Scholar]
- [36].Myers BD, Ross J, Newton L, Luetscher J, Perlroth M, Cyclosporine-associated chronic nephropathy, New England Journal of Medicine, 311 (1984) 699–705. [DOI] [PubMed] [Google Scholar]
- [37].Hyon JY, Lee YJ, Yun P-Y, Management of ocular surface inflammation in Sjögren syndrome, Cornea, 26 (2007) S13–S15. [DOI] [PubMed] [Google Scholar]
- [38].Dastjerdi MH, Hamrah P, Dana R, High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen, Cornea, 28 (2009) 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou XQ, Wei RL, Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis, Cornea, 33 (2014) 760–767. [DOI] [PubMed] [Google Scholar]
- [40].Keating SM, Clark KR, Stefanich LD, Arellano F, Edwards CP, Bodary SC, Spencer SA, Gadek TR, Marsters JC, Beresini MH, Competition between intercellular adhesion molecule1 and a small‐molecule antagonist for a common binding site on the αl subunit of lymphocyte function‐associated antigen‐1, Protein science, 15 (2006) 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA, Small molecule integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in the other, Immunity, 19 (2003) 391–402. [DOI] [PubMed] [Google Scholar]
- [42].Aliabadi HM, Mahmud A, Sharifabadi AD, Lavasanifar A, Micelles of methoxy poly (ethylene oxide)-b-poly (ɛ-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A, Journal of Controlled Release, 104 (2005) 301–311. [DOI] [PubMed] [Google Scholar]
- [43].Smeesters C, Giroux L, Vinet B, Arnoux R, Chaland P, Corman J, St-Louis G, Daloze P, Efficacy of incorporating cyclosporine into liposomes to reduce its nephrotoxicity, Canadian journal of surgery. Journal canadien de chirurgie, 31 (1988) 34–36. [PubMed] [Google Scholar]
- [44].Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S, Advances and challenges of liposome assisted drug delivery, Frontiers in pharmacology, 6 (2015) 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Szebeni J, Barenholz Y, Adverse immune effects of liposomes: complement activation, immunogenicity and immune suppression, Harnessing Biomaterials for Nanomedicine: Preparation, Toxicity and Applications, ed PS Publishing (Singapore: Pan Stanford Publishing; ), (2009) 1–19. [Google Scholar]
- [46].Forssen E, Willis M, Ligand-targeted liposomes, Advanced drug delivery reviews, 29 (1998) 249–271. [DOI] [PubMed] [Google Scholar]
- [47].Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R, Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial, Blood, 73 (1989) 1729–1734. [PubMed] [Google Scholar]
- [48].Storb R, Deeg H, Farewell V, Doney K, Appelbaum F, Beatty P, Bensinger W, Buckner C, Clift R, Hansen J, Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease, Blood, 68 (1986) 119–125. [PubMed] [Google Scholar]
- [49].Malingré MM, Richel DJ, Beijnen JH, Rosing H, Koopman FJ, Huinink W.W. Ten Bokkel, Schot ME, Schellens JH, Coadministration of cyclosporine strongly enhances the oral bioavailability of docetaxel, Journal of Clinical Oncology, 19 (2001) 1160–1166. [DOI] [PubMed] [Google Scholar]
- [50].Nakahara C, Nakamura K, Yamanaka N, Baba E, Wada M, Matsunaga H, Noshiro H, Tanaka M, Morisaki T, Katano M, Cyclosporin-A enhances docetaxel-induced apoptosis through inhibition of nuclear factor-κB activation in human gastric carcinoma cells, Clinical cancer research, 9 (2003) 5409–5416. [PubMed] [Google Scholar]
- [51].Shi P, Aluri S, Lin Y-A, Shah M, Edman M, Dhandhukia J, Cui H, MacKay JA, Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo, Journal of controlled release, 171 (2013) 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dhandhukia JP, Li Z, Peddi S, Kakan S, Mehta A, Tyrpak D, Despanie J, MacKay JA, Berunda Polypeptides: Multi-Headed Fusion Proteins Promote Subcutaneous Administration of Rapamycin to Breast Cancer In Vivo, Theranostics, 7 (2017) 3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A, Self-assembling chimeric polypeptide–doxorubicin conjugate nanoparticles that abolish tumours after a single injection, Nature materials, 8 (2009) 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bidwell GL III, Fokt I, Priebe W, Raucher D, Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin, Biochemical pharmacology, 73 (2007) 620–631. [DOI] [PubMed] [Google Scholar]
- [55].Bhattacharyya J, Bellucci JJ, Weitzhandler I, McDaniel JR, Spasojevic I, Li X, Lin C-C, Chi J-TA, Chilkoti A, A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models, Nature communications, 6 (2015) 7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Boumba D, Skopouli F, Moutsopoulos H, Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome, Rheumatology, 34 (1995) 326–333. [DOI] [PubMed] [Google Scholar]
- [57].Roescher N, Tak PP, Illei GG, Cytokines in Sjögren’s syndrome, Oral diseases, 15 (2009) 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ye C, Brand D, Zheng SG, Targeting IL-2: an unexpected effect in treating immunological diseases, Signal Transduction and Targeted Therapy, 3 (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Boyman O, Sprent J, The role of interleukin-2 during homeostasis and activation of the immune system, Nature Reviews Immunology, 12 (2012) 180. [DOI] [PubMed] [Google Scholar]
- [60].Tang Q, Therapeutic window of interleukin-2 for autoimmune diseases, Diabetes, 64 (2015) 1912–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang C, Zhang J, Yang B, Wu C, Cyclosporin A inhibits the production of IL-17 by memory Th17 cells from healthy individuals and patients with rheumatoid arthritis, Cytokine, 42 (2008) 345–352. [DOI] [PubMed] [Google Scholar]
- [62].Roescher N, Tak PP, Illei GG, Cytokines in Sjögren’s syndrome: potential therapeutic targets, Annals of the rheumatic diseases, 69 (2010) 945–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM, Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis, The American journal of pathology, 175 (2009) 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ, Sjöstrand M, Eloranta ML, Gabhann JN, Winqvist O, Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23–Th17 pathway, Journal of experimental medicine, 206 (2009) 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S, Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18, The Journal of Immunology, 181 (2008) 2898–2906. [DOI] [PubMed] [Google Scholar]
- [66].Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB, Salivary gland tissue expression of interleukin‐23 and interleukin‐17 in Sjögren’s syndrome: Findings in humans and mice, Arthritis & Rheumatology, 58 (2008) 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.