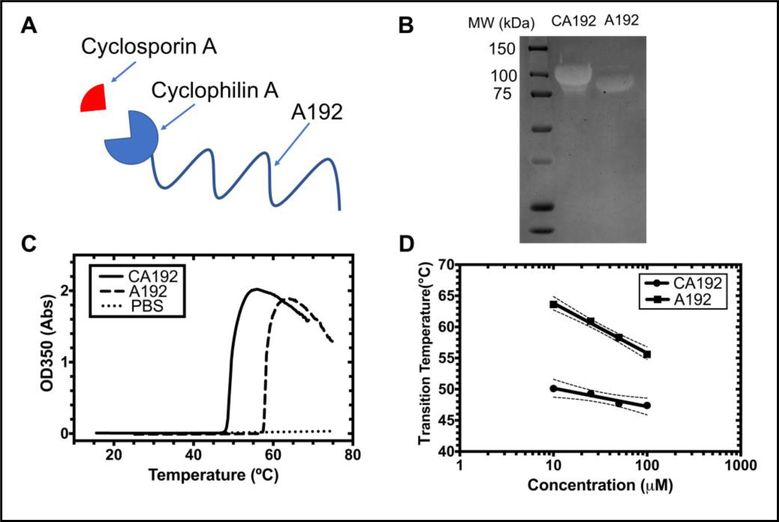
Figure 1. A recombinant elastin-like polypeptide (ELP) fusion protein as a non-covalent drug carrier for cyclosporine A (CsA).
A) A gene was constructed encoding the cyclophilin A protein fused to a high molecular weight ELP, A192. This fusion, called CA192, binds specifically and with high affinity to CsA; B) SDS-PAGE of purified CA192 (91.6 kDa) stained with copper chloride demonstrates a molecular weight shift upon fusing CypA to A192 (73.6 kDa); C) Optical density was determined as a function of temperature (1°C/min) for 25 μM CA192, free A192, and a PBS control. The temperature with the maximum positive slope was defined as the phase transition temperature. D) The concentration-temperature phase diagrams of both CA192 and A192 are plotted, showing that both are expected to remain soluble at physiological temperatures. The 95% confidence interval around each best-fit line is indicated with dashed lines.