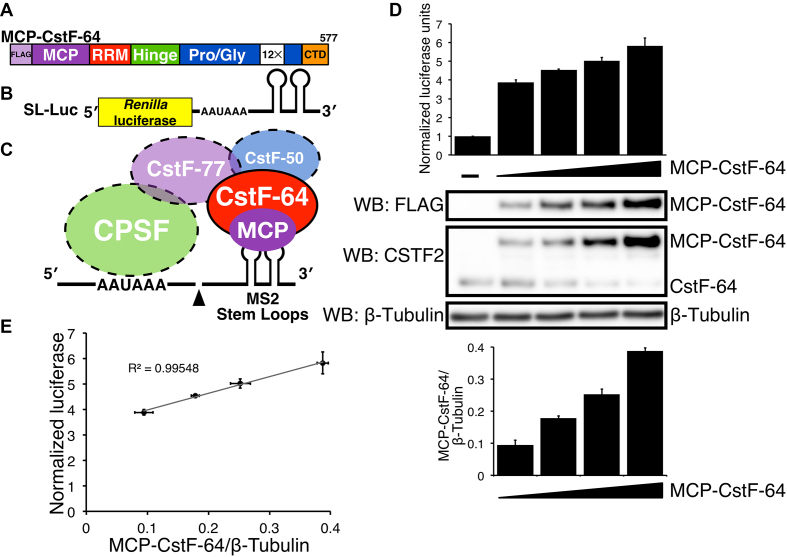
Figure 1.
CstF-64 stimulates cleavage and polyadenylation in correlation to the amount of CstF-64 protein produced in SLAP. (A) Schematic representation of the coat binding protein from bacteriophage MS2 (MCP) fused to human CstF-64 (MCP-CstF-64). Functional domains in CstF-64 and three FLAG tags are indicated. (B) Schematic representation of the reporter construct (SL-Luc) containing the Renilla luciferase and two MS2 stem-loop (SL) sequences downstream of the cleavage and polyadenylation site. (C) The interaction between MCP-CstF-64 and the SL-Luc reporter construct is mediated through the interaction between MCP and MS2 stem-loops. (D) SLAP results showing NLUs in HeLa cells without MCP-CstF-64 (–), and in cells transfected with increasing amounts of MCP-CstF-64. Western blots with the designated antibodies to show the expression of the FLAG-tagged version of MCP-CstF-64 (WB: FLAG) and endogenous CstF-64 (WB: CSTF2). β-tubulin was used as a loading control. A bar graph of the quantified expression of MCP-CstF-64 normalized to β-tubulin (bottom). (E) A linear regression between the amount of MCP-CstF-64 protein normalized to β-tubulin (x-axis) to the NLU from SLAP (y-axis). The correlation value (R2 = 0.99548) is shown.