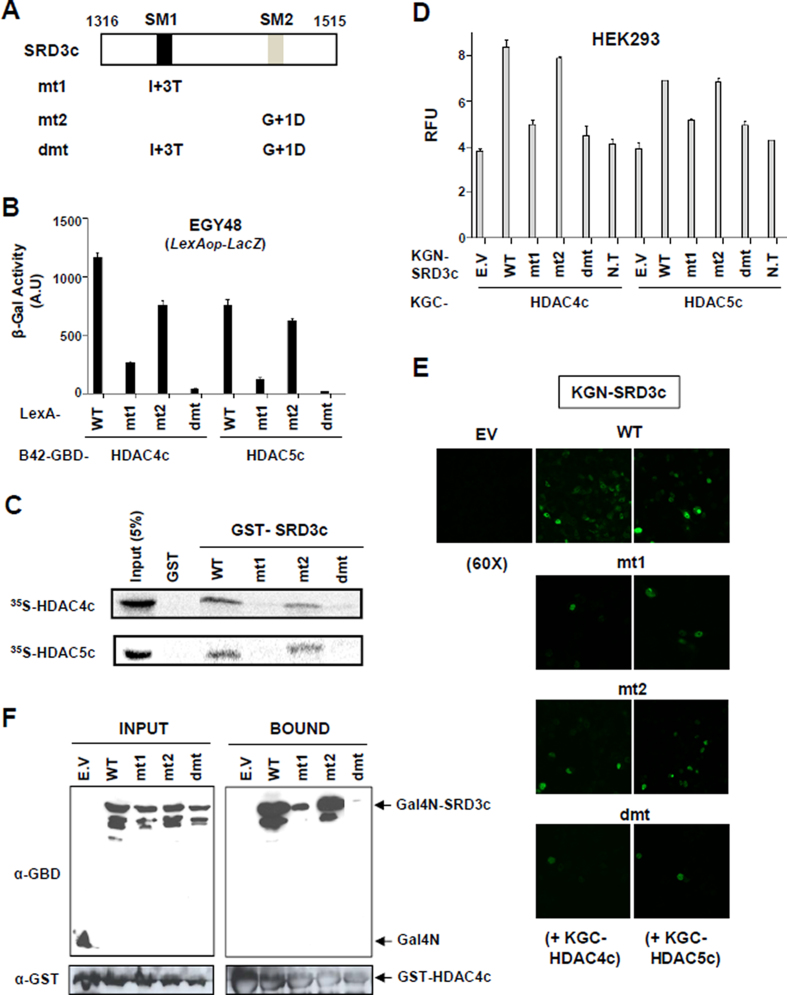
Figure 2.
SM1 is more important than SM2 for the interaction between SRD3c and HDAC4c/-5c. (A) Schematic diagram of SRD3c mutants. The first glycine residue of the SM1 (GSITQGIPR) or SM2 (GSITQGTPL) is denoted as +1. Single amino acid substitution is introduced into SM1 (I+3T) or SM2 (G+1D) motif of SRD3c and denoted as mt1 or mt2, respectively. The dmt indicates double-mutant containing both mt1 and mt2 mutations. (B) Yeast two-hybrid assay. Yeast strain EGY48 bearing lexAop-LacZ reporter was co-transformed with expression plasmids for LexA-fused SRD3c derivatives (wild-type, mt1, mt2, or dmt) and B42AD-GBD fusions of HDAC4c/-5c. Transformants were grown in synthetic minimal glucose media, and liquid β-galactosidase assays were performed. WT: wild-type. (C) In vitro GST pull-down assay. GST or GST-SRD3c derivatives were tested for interactions with in vitro synthesized HDAC4c/-5c proteins. Input represents 5% of the in vitro translated HDAC4c/-5c used in the pull-down assays. (D) Quantitative BiFC assay in HEK293 cells. Fluorescence signals generated via the interactions between indicated KGN-SRD3c proteins and KGC-HDAC4c/-5c were spectrophotometrically measured as described in Materials and methods. (E) Confocal laser-scanning image for the BiFC interaction assay between indicated KGN-SRD3c proteins and KGC-HDAC4c/-5c. Magnification: 60 X. (F) In vivo GST pull-down assay. HEK293 cells were transiently transfected with expression vectors for GST-HDAC4c and indicated Gal4N-SRD3c derivatives. Whole-cell extracts were prepared and incubated with glutathione-Sepharose beads. The bound proteins were analyzed via immunoblotting using anti-GBD (for Gal4N) and anti-GST antibodies. E.V: empty vector, N.T: no transfection.