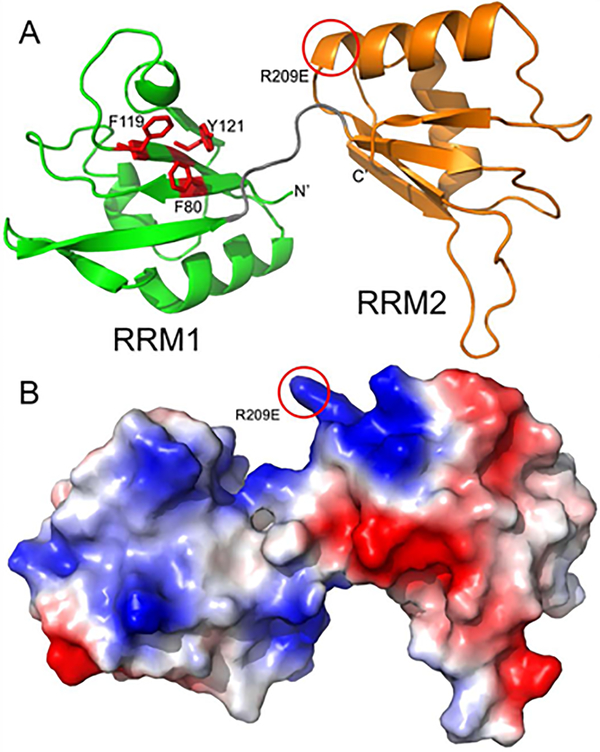
Figure 6. Homology modeling of Xenopus laevis Dnd1 suggests that only the N-terminal RRM domain is likely to bind RNA.
A. Homology model of Dnd1 RRM domains is shown in a ribbon representation with RRM1 (residues 78–151) colored green and RRM2 (residues 158–233) colored orange. The linker between the two RRM domains is shown in grey, and the relative orientation between the two domains is likely variable and dependent on parts of Dnd1 not included in this model. RRM1 domain of Dnd1 has three aromatic residues (shown as red sticks) poised for stacking with RNA, while RRM2 has no aromatic side chains facing out from its beta sheet. Location of putative ATP binding on RRM2 is highlighted by a red circle. B. Homology model from panel A, shown as a molecular surface colored by electrostatic potential (red is acidic, blue is basic).