Figure 4: LKB1 phosphorylates PRMT5 on threonine residues.
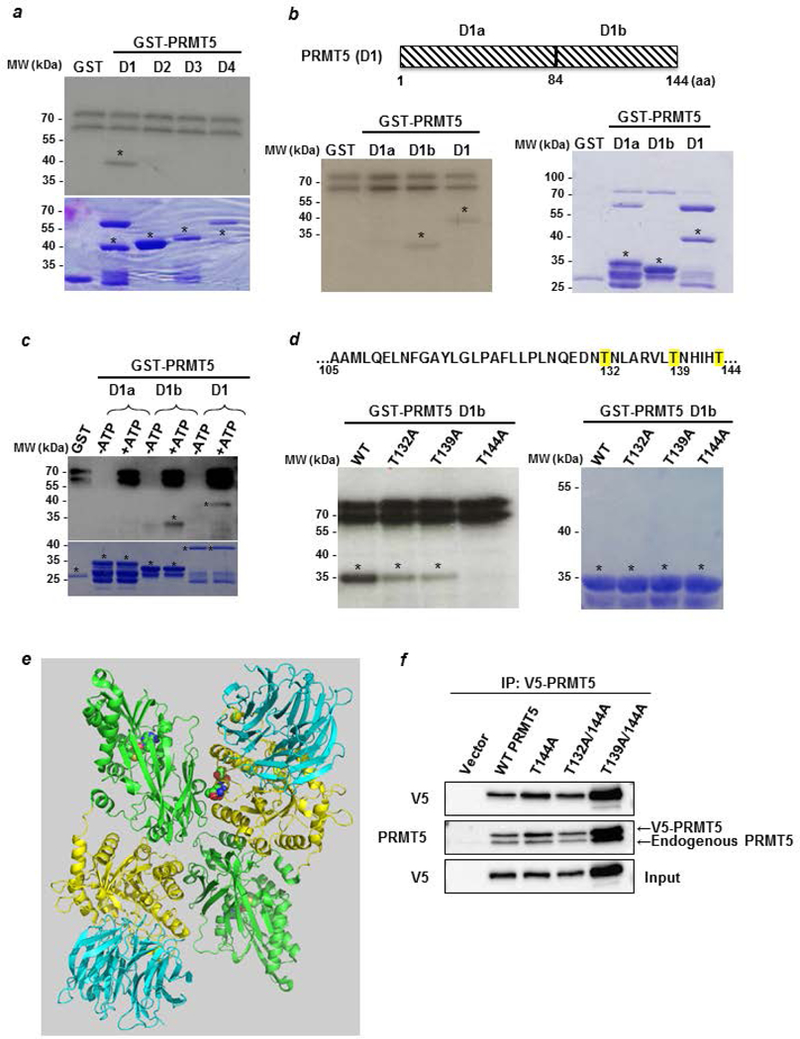
(a) An in vitro phosphorylation experiment was performed by incubating active LKB1 complex with [32Pγ] ATP and D1 to D4 PRMT5 fragments fused to GST. The phosphorylated proteins were visualized by autoradiography (upper panel). The corresponding Coomassie-stained gel is shown in the lower panel. * indicates the full length fusion proteins. (b) The PRMT5 D1 domain was divided into two parts, D1a and D1b, which were used to perform an in vitro kinase assay as described in a (left panel). The corresponding Coomassie-stained gel is shown in the right panel. * indicates the full length fusion proteins. (c) Cold in vitro phosphorylation experiments were performed by incubating active LKB1 complex with D1a, D1b and D1 PRMT5 fragments fused to GST in the absence or the presence of ATP. The phosphorylated proteins were verified by Western blot analysis using a pan phospho-Thr antibody (upper panel). The corresponding Coomassie-stained gel is shown in the lower panel. * indicates the full length fusion proteins. (d) Sequence of the PRMT5 D1b containing the potential sites for LKB1 phosphorylation. T132, 139 and 144, are highlighted in yellow. GST-D1b wild type or T/A mutants were used as substrates for radioactive LKB1 phosphorylation (left panel). The corresponding Coomassie-stained gel is shown in the right panel. * indicates the full length fusion proteins. (e) Structure of human PRMT5/MEP50 heterodimer. The N-terminal of each monomer, containing the TIM-Barrel domain, is represented in yellow and its C-terminal domain in green. The 3 mutated threonines are in the spacefill at the dimer interface. The protein represented in blue is MEP50. (Based on PDB 5fa5 from (39) and represented with the PyMOL molecular graphics system, version 2.0, Schrödinger, LLC). (f) MCF-7 cells were transfected with pcDNA3.1 V5-PRMT5 WT and mutants for 48 hr. Cell lysates analyzed for V5-PRMT5 expression with an anti-V5 antibody. Then, V5-PRMT5 was immunoprecipitated using an anti-V5 antibody and revealed by Western blot with the anti-V5 and anti-PRMT5 antibodies.
