Figure 4.
Rainbow Analysis by Flow Cytometry Faithfully Reports Levels of 3TF Transgenic Expression
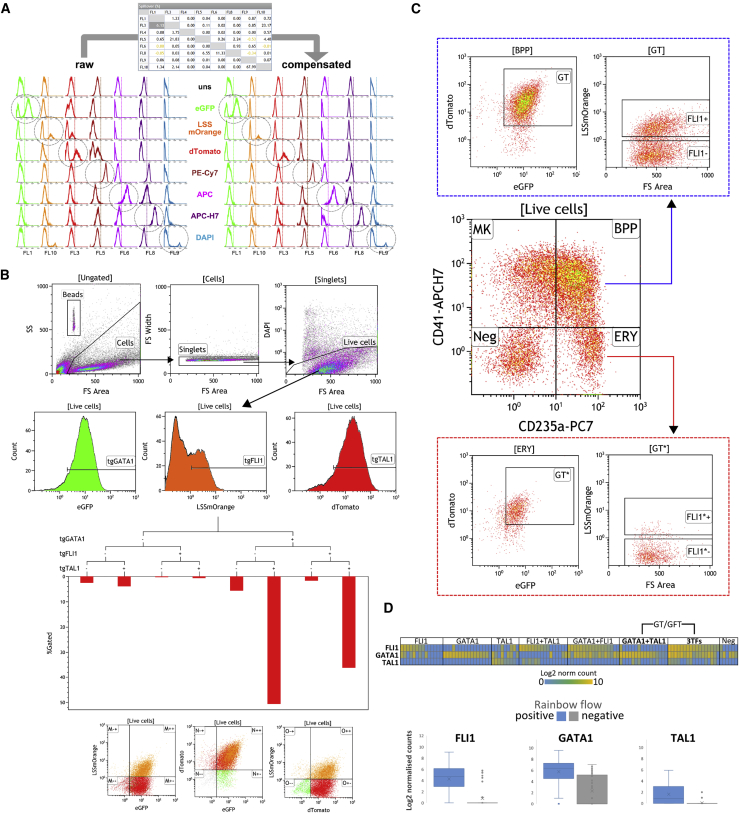
Human iPSCs were transduced with the three rainbow vectors designed to report transgene expression and analyzed by multicolor flow cytometry for expression of eGFP(GATA1), LSSmOrange(FLI1), and dTomato(TAL1), in addition to the surface markers Pe-Cy7(CD235a), APC(CD309/CD42a), and APC-H7(CD41), and the DNA stain DAPI (viability). Representative data shown from the FFDK cell line at day 9 post-transduction.
(A) Single-color control samples were run alongside each flow experiment to set up a compensation matrix efficiently correcting for spillover between optical channels.
(B) Gating strategy for analysis of the rainbow sub-populations. Single-color histograms were used to read each rainbow color separately from the single live cell gate. The statistics for the eight unique transgenic populations were then collected using the radar plot capacity of the Beckman Coulter Kaluza software.
(C) Flow cytometry sorting gating strategy for day10 cells. The [BPP] population was defined from the single live cell gate as cells co-expression the surface markers CD41 and CD235. The [BPP] population was further gated for cells co-expressing the reporters eGFP(GATA1) and dTomato(TAL1) further split into LSSmOrange(FLI1) positive and negative cells: this led to the collection of the [BPP-GFT] and [BPP-GT] populations, respectively, for the downstream analyses described in the manuscript. Illustration of the rainbow expression profile in the [ERY] population is also shown as a contrasting profile.
(D) The expression of the transgenic TFs from flow-sorted populations based on rainbow reporter expression was further analyzed at the transcript level and single-cell resolution by RNA sequencing. The Log2 normalized transgenic read counts from the eight possible rainbow combinations are shown as a heatmap (n = 111 cells). The boxplots show pooled data from rainbow-positive (blue) or -negative (gray) cells taking in consideration a single rainbow reporter at a time from the same dataset.