Fig. 1.
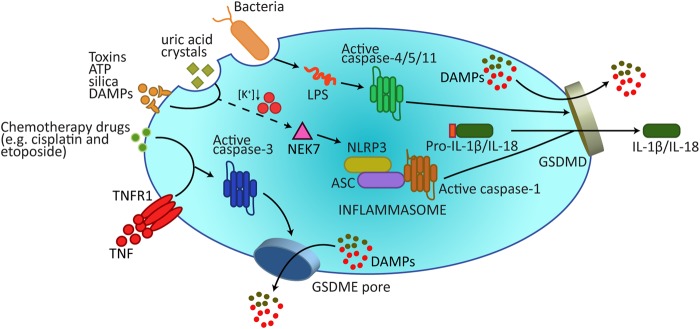
Gasdermins form membrane pores to cause pyroptosis. A wide array of extracellular stimuli can drive pyroptosis. In the canonical model of pyroptosis, inflammasome sensor proteins, such as NLRP3, recognize cellular stressors, including those from bacteria, viruses, toxins, ATP, uric acid crystals, silica, and DAMPs. These stressors activate NLRP3 indirectly through potassium efflux, which leads to NEK7 binding NLRP3 to trigger its oligomerization. NLRP3 subsequently activates caspase-1 via the adaptor protein ASC. Caspase-1 processes and activates IL-1β and IL-18, and also cleaves GSDMD to release the membrane pore-forming GSDMD-N domain. GSDMD-N pores promote the release of activated IL-1β and IL-18 and, most likely, DAMPs that can be accommodated by the 10–20 nm pore diameter. Additional DAMPs will be released following the collapse of the plasma membrane. Cytosolic LPS binds Caspase-4/5/11 to trigger their cleavage of GSDMD, but not IL-1β and IL-18. In addition, recent research has revealed how the apoptotic effector caspase, caspase-3, can cleave GSDME to also cause pyroptotic death. DAMPs damage-associated molecular patterns, LPS lipopolysaccharide