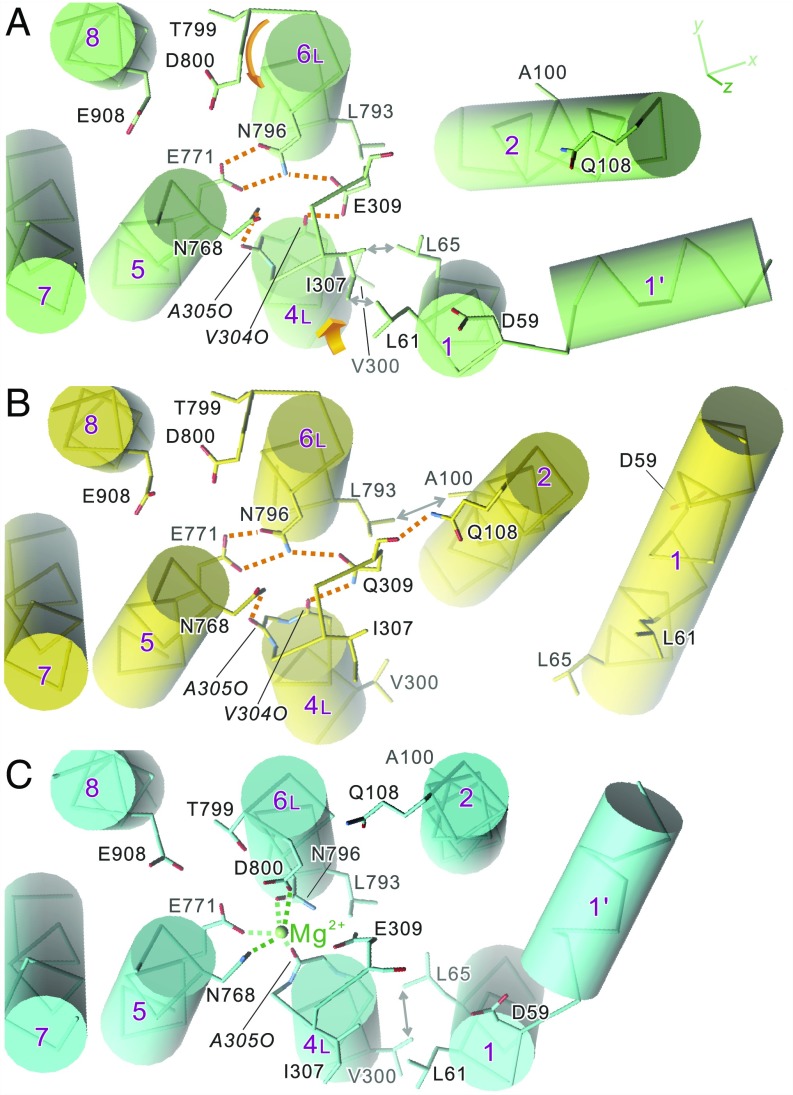
Fig. 3.
Disposition of the transmembrane helices in crystal structures of SERCA1a: native ATPase in E2(TG) (A), Glu309Gln mutant (B), and native ATPase in E1·Mg2+ (C). Viewed from the cytoplasmic side approximately perpendicular to the membrane. Orange dotted lines represent likely hydrogen bonds, and those in green represent Mg2+ coordination. Orange arrows in A indicate the directions to which the unwound part of M6 rotates and M4L moves in the E2 → E1·Mg2+ transition. Note that dispositions of the side chains of the Ca2+-coordinating residues (Glu309 on M4; Asn768 and Glu771 on M5; Asn796, Thr799, and Asp800 on M6; and Glu908 on M8) in the Glu309Gln mutant (B) are exactly the same as those in native enzyme (A), although the M1 and M2 helices occupy distinctly different positions. Double-headed arrows indicate van der Waals contacts.