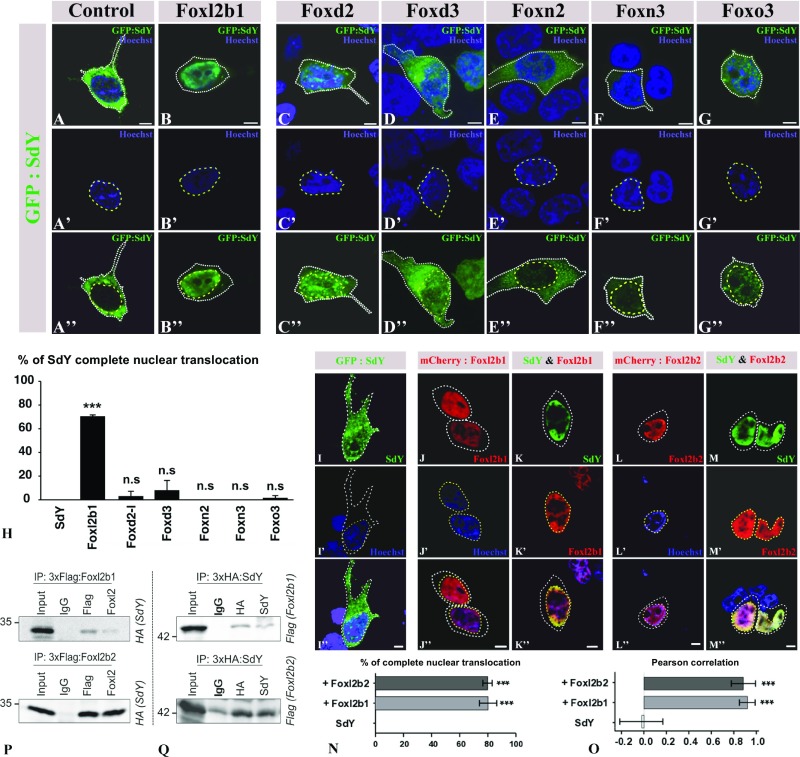
Fig. 2.
SdY interacts with Foxl2, resulting in its nuclear translocation. (A–H) GFP:SdY alone (A–A″) and GFP:SdY in combination with different trout Fox proteins, Foxl2 (Foxl2b1) (B–B″), Foxd2 (C–C″), Foxd3 (D–D″), Foxn2 (E–E″), Foxn3 (F–F″), Foxo3 (G–G″), were cotransfected in HEK 293T cells (delimited by white dotted lines). GFP:SdY is translocated in the nucleus (delimited by yellow dotted lines and stained in blue with Hoechst staining) only in the presence of Foxl2 (B–B″). (Scale bar, 5 μm.) (H) Percentage of transfected cells (mean ± standard deviation on 200 cells) in which SdY is completely translocated in the nucleus after three independent cotransfection experiments with different trout Fox proteins. Significant differences compared with SdY alone were calculated using an unpaired two-tailed Student’s t test, ***P < 0.001; ns, nonsignificant. (I–O) Foxl2b1 and Foxl2b2 are both able to drive SdY complete nuclear translocation (delimited by yellow dotted lines and stained in blue with Hoechst staining). Confocal images of HEK 293T cells (delimited by white dotted lines) transiently transfected with sdY (I–I″), mCherry:Foxl2b1 alone (J–J″), SdY and mCherry:Foxl2b1 (K–K″), mCherry:Foxl2b2 alone (L–L″), SdY and mCherry:Foxl2b2 (M–M″). (Scale bar, 10 µm.) (N) Quantitative analysis in the presence or absence of Foxl2b1 and Foxl2b2. Percentage of complete SdY nuclear translocation (mean ± standard deviation on 100 cells) after three independent cotransfection experiments. Statistical significances compared with SdY alone were calculated using an unpaired two-tailed Student’s t test. (O) SdY colocalizes with Foxl2 in the nucleus. SdY, SdY-Foxl2b1, and SdY-Foxl2b2 colocalizations were measured in the nucleus for SdY (n = 5), SdY and Foxl2b1 (n = 5), and SdY and Foxl2b2 (n = 5) with Pearson’s correlation. Statistical significance was calculated using an unpaired two-tailed Student’s t test, ***P < 0.001. (P and Q) SdY binds with Foxl2 in co-immunoprecipitation (IP) experiments. HEK 293T cells were transiently transfected with expression plasmids for SdY fused to a hemagglutinin tag (3xHA:SdY) and for Foxl2 fused to a 3xFlag tag (3xFlag:Foxl2b1 or 3xFlag:Foxl2b2). Whole-cell lysates were used for immunoprecipitation with anti-Flag or anti-Foxl2 (P) and with anti-HA or anti-SdY (Q) followed by immunoblotting with the appropriate antibodies. Input represents 10% whole-cell lysate. IgG mouse antibody was used as the control. In P, 3xFlag:Foxl2b1 or 3xFlag:Foxl2b2 was immunoprecipitated with either Flag (Top) or FoxL2 (Bottom) antibodies followed by immunoblotting with an antibody against the HA tag to reveal the interaction with 3xHA:SdY (SdY). In Q, 3xHA:SdY was immunoprecipitated with an HA or SdY antibody, followed by immunoblotting with an antibody against the Flag tag to reveal 3xFlag:Foxl2b1 (Foxl2b1) (Top) or 3xFlag:Foxl2b2 (Foxl2b2) (Bottom).