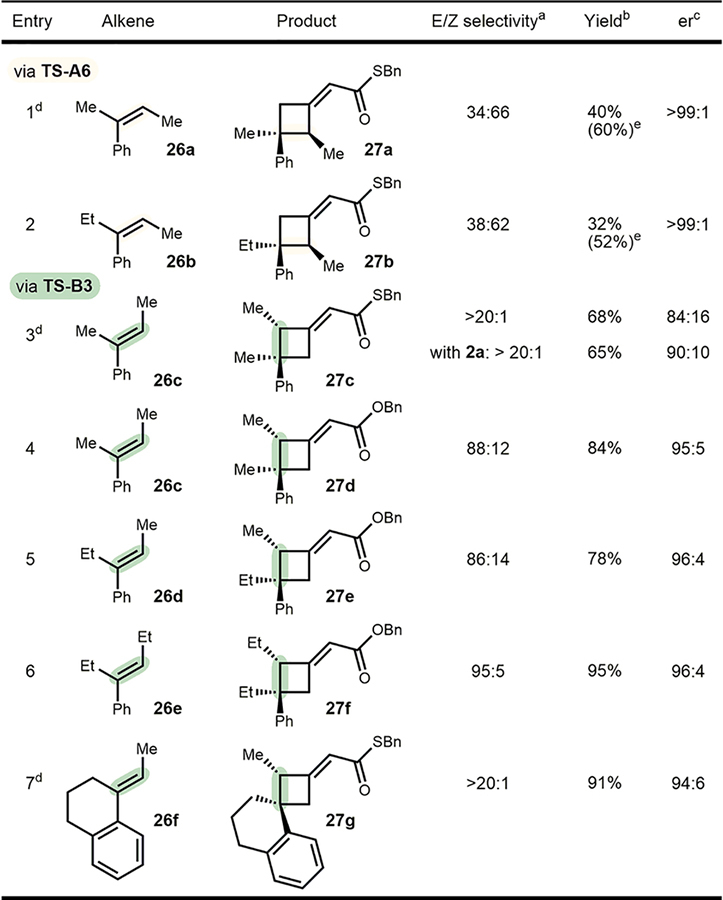
Table 2.
Stereospecific [2+2] Cycloadditions
|
Determination by 1H NMR of the crude reaction mixture utilizing a calibrated standard. Reactions run under optimized conditions using catalyst 2e.
Yields reported of pure major isomer as average of two experiments.
Enantiomeric ratio of the major isomer (see Supporting Information for er of minor isomers).
Reactions run at −20 °C.
Combined yield of both isomers in parentheses.
