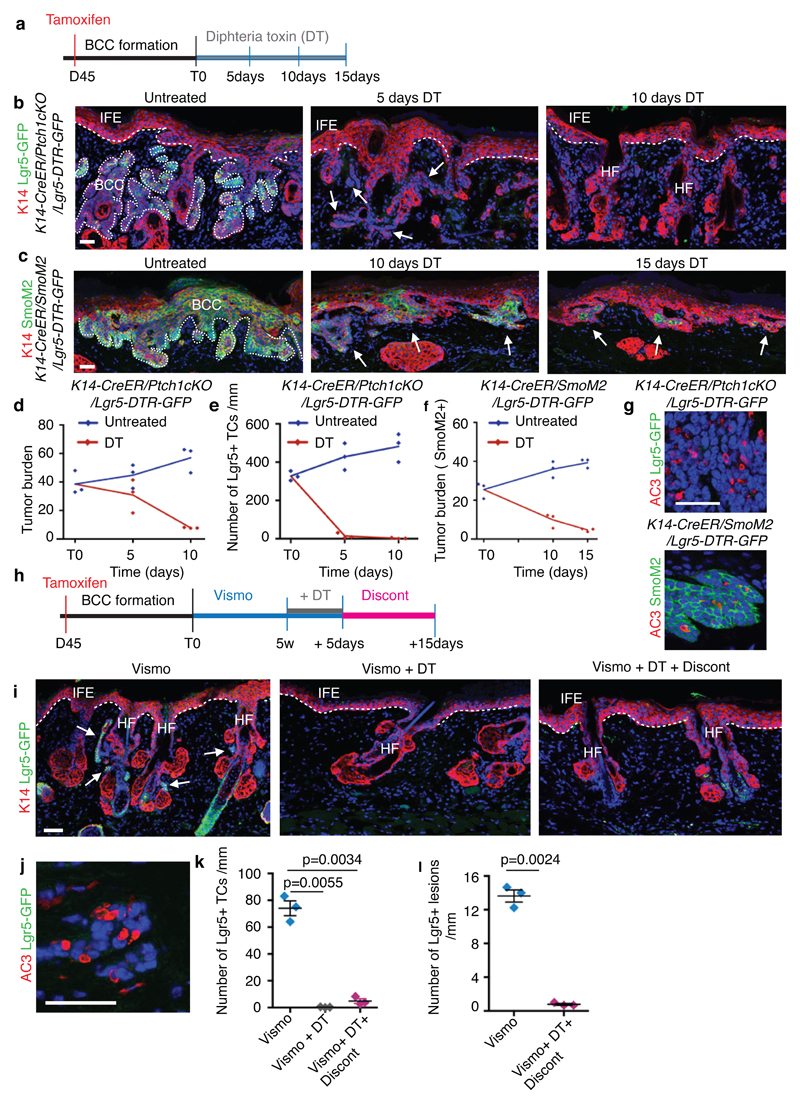
Extended Data Fig. 9. Lgr5 lineage ablation leads to BCC shrinkage and elimination of vismodegib-persistent lesions.
(a) Protocol for tamoxifen and diphtheria toxin (DT) administration. (b,c) Immunostaining for K14 and GFP in the Ptch1cKO/Lgr5-DTR-GFP model (b) and for K14 and SmoM2 in SmoM2-model (c) at different time points upon DT administration. (d) Quantification of tumour burden in untreated and following DT administration (n=3 mice per time point and condition). Centre values define the mean. Description of the skin length and tumour area analysed per mouse in Source Data.(e) Number of GFP positive TCs in untreated conditions and following DT administration (n=3 Ptch1cKO/Lgr5-DTR-GFP mice per time point and condition, 1mm of skin analysed per mouse).Centre values define the mean.(f) Quantification of tumour burden (SmoM2-expressing cells) in untreated conditions and following (n=3 mice per time point and condition). Centre values define the mean. Description of the skin length and tumour area analysed per mouse in Source Data.(g) Immunostaining for active caspase3 (AC3) and GFP (upper panel) and for active caspase-3 and SmoM2 (lower panel) after 5 times of DT administration. Three independent experiments per condition were analysed showing similar results. (h) Experimental strategy for combination of vismodegib treatment and Lgr5 ablation in K14-CreER/Ptch1cKO/Lgr5-DTR-GFP mice.(i) Immunostaining for GFP and K14 in the upon treatment, Lgr5 ablation and discontinuation in Ptch1cKO/Lgr5-DTR-GFP mice. (j) Immunostaining for active caspase-3 and GFP following vismodegib+DT administration in Ptch1cKO/Lgr5-DTR-GFP mice. (k) Quantification of the number of GFP positive cells in the different experimental conditions upon treatment and discontinuation (n=3 Ptch1cKO/Lgr5-DTR-GFP mice, 3mm of skin analysed per mouse). Mean+/-s.e.m. Two-sided t-test. (l) Quantification of the number of Lgr5+ lesions per length of epidermis (mm) in mice treated with vismodegib and upon vismodegib +DT treatment discontinuation (n=3 Ptch1cKO/Lgr5-DTR-GFP mice, 3mm of skin analysed per mouse). Mean+/-s.e.m. Two-sided t-test. Hoechst nuclear staining in blue; scale bars, 50 μm. IFE: interfollicular epidermis, BCC: basal cell carcinoma, HF: hair follicle. Dashed line delineates basal lamina separating IFE from the dermis. Dotted line delineates BCC. Arrow indicates tumorigenic lesions in b, c and indicates vismodegib-persistent lesion in i.