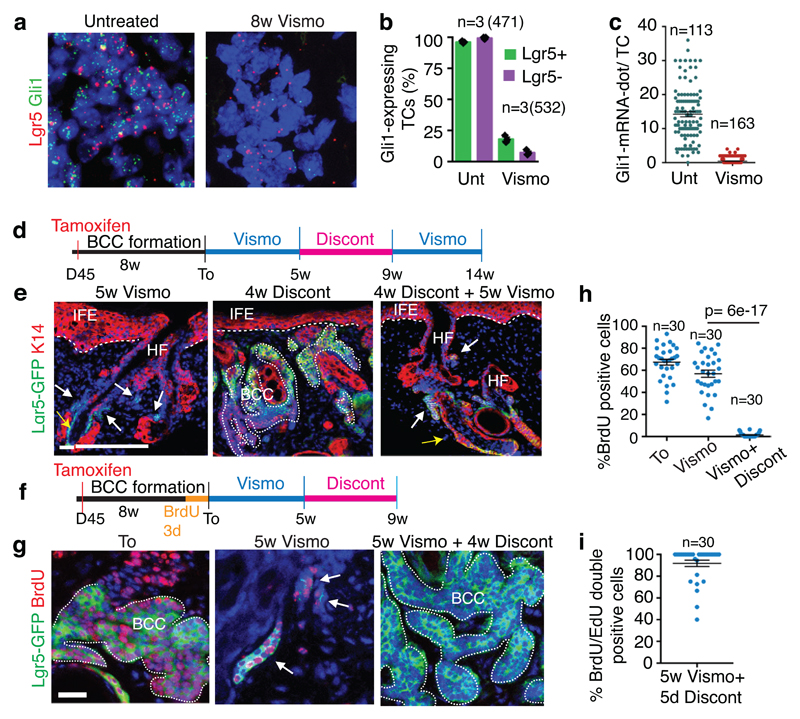
Fig. 2. Slow-cycling Lgr5+ LRCs mediate tumour relapse following vismodegib discontinuation.
(a) In situ hybridization for Lgr5 and Gli1 in untreated and treated Ptch1cKO-induced BCCs. (b) Percentage of TCs (Lgr5+ and Lgr5-) that express Gli1 (n=3 Ptch1cKO mice, total number of cells analysed indicated in parenthesis). Mean +/- s.e.m. (c) Distribution of the number of Gli1-mRNA dot per TC with and without treatment (n=113 and 163 total TCs from 3 mouse per condition and time point). Mean +/- s.e.m. (d) Protocol for vismodegib administration, discontinuation and re-administration (e) Immunostaining for Lgr5-DTR-GFP (Lgr5-GFP) and Keratin-14 (K14) in Ptch1cKO ventral skin following vismodegib treatment, discontinuation and vismodegib re-administration. 3 independent experiments per condition were analysed showing similar results.(f) Protocol for BrdU pulse chase label retention studies followed by vismodegib administration and discontinuation. (g) Immunostaining for Lgr5-GFP and BrdU following BrdU administration and upon BrdU chase in Ptch1cKO-induced BCCs. (h-i) Proportion of Lgr5+LRCs at T0, after vismodegib treatment and discontinuation (h) and BrdU/EdU double positive TCs 5 days after vismodegib discontinuation (i) in Ptch1cKO/Lgr5-DTR-GFP-derived BCCs (n=30 lesions analysed from 3 mice per condition in h and i). Mean +/- s.e.m. Two-sided t-test. Hoechst nuclear staining in blue; scale bars, 25 μm. Dashed line delineates basal lamina. White arrow indicates vismodegib-persistent lesions and yellow arrow indicates HF Lgr5+ cells.