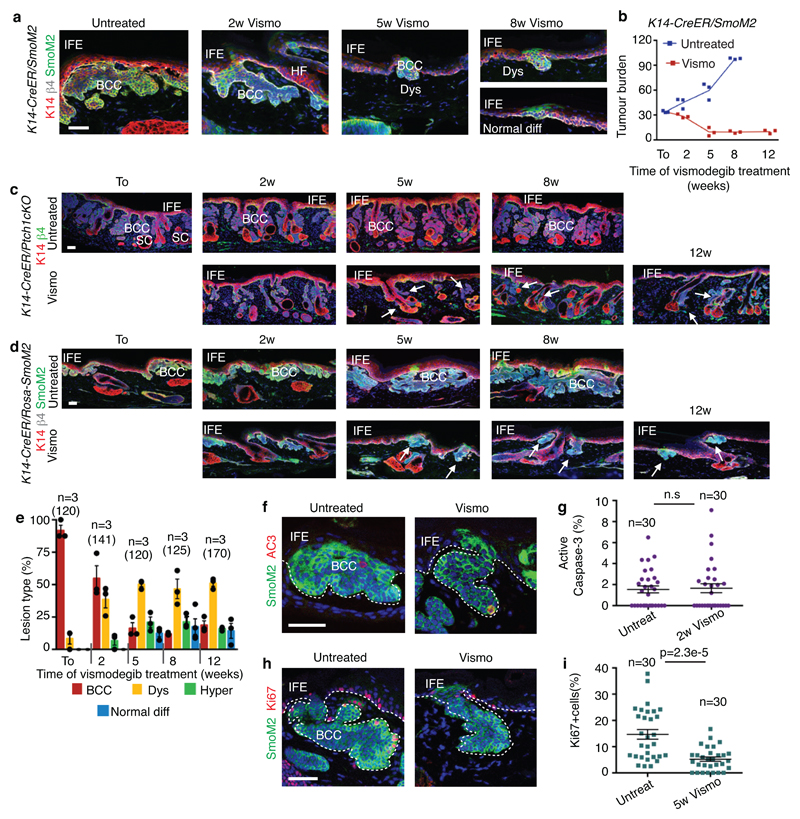
Extended Data Fig 1. Vismodegib leads to tumour shrinkage and emergence of vismodegib-persistent lesions in mice.
(a) Immunostaining for SmoM2-YFP, K14 and β4-integrin in tail from SmoM2 mice at different time points following vismodegib administration. (b) Tumour burden in μm (total area occupied by tumours divided by the length of the analysed epidermis) in untreated and vismodegib-treated SmoM2 mice (n=3 mice analysed per time point and condition). Centre values define the mean. Description of the skin length and tumour area analysed per mouse in Source Data.(c) Immunostaining for K14 and β4-integrin in ventral skin from Ptch1cKO mice (d) Immunostaining for SmoM2, Keratin-14 and β4-integrin in tail skin from SmoM2 mice.(e) Quantification of the lesion type upon vismodegib treatment in SmoM2 mice (n= 3 mice, total number of lesions analysed per time point indicated in parenthesis). Histograms represent the mean and error bars the s.e.m. (f) Immunostaining for active caspase-3 (AC3) and SmoM2. (g) Percentage of AC3+ TCs in untreated and vismodegib-treated SmoM2 mice (n=30 lesions analysed from 3 mice). Mean +/- s.e.m. Two-sided t-test.(h) Immunostaining for Ki67 and SmoM2. (i) Percentage of Ki67+ TCs in untreated and vismodegib-treated SmoM2 mice (n=30 lesions analysed from 3 mice). Mean +/- s.e.m. Two-sided t-test. Three independent experiments per condition were analysed showing similar results in a, c, d, f and h. Hoechst nuclear staining in blue; scale bars, 100 μm in c and d, 50 μm in a, f and h. IFE: interfollicular epidermis, BCC: basal cell carcinoma, HF: hair follicle, Dys: dysplasia. Dashed line delineates basal lamina. Arrows indicate vismodegib-persistent lesions.