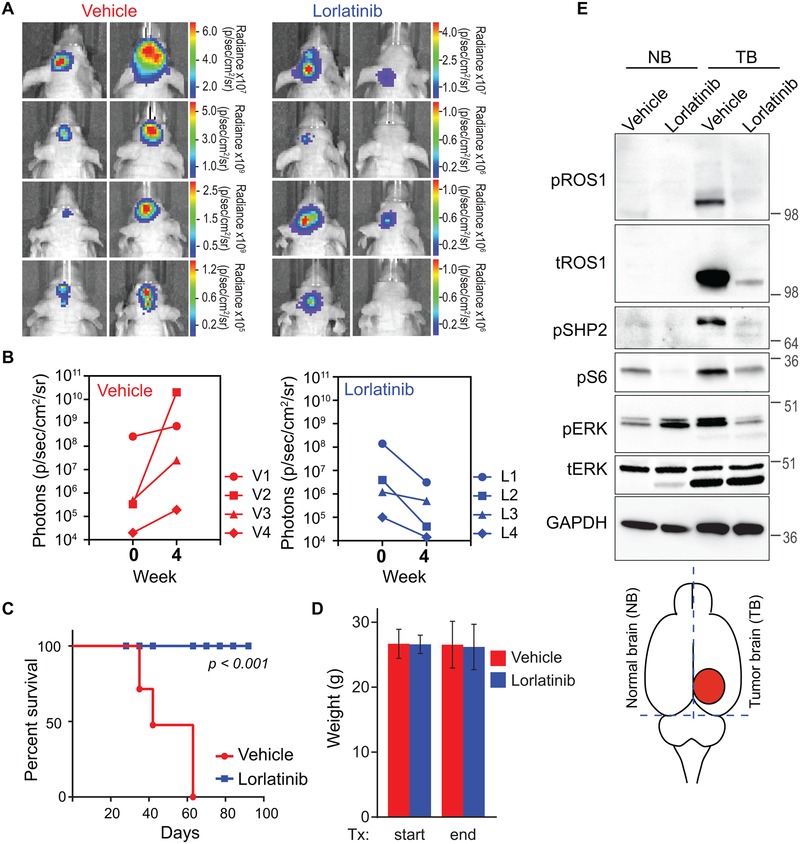
Figure 5. Oral monotherapy with lorlatinib decreases tumor burden and prolongs survival in an intracranial U118MG GBM xenograft model.
A. Bioluminescence imaging of the U118MG-xenografted tumors pre- and post-four weeks of Vehicle or lorlatinib treatment (30 mg/kg by oral gavage, once daily). Firefly luciferase-labeled U118MG GBM cells had been implanted into forebrain of NOD-scid mice four weeks prior to starting treatment. B. Photon emission as surrogate readout for tumor volume at start of treatment (week 0) and end of treatment (week 4) in Vehicle treated (left graph) and lorlatinib-treated (right graph) mice. C. Vehicle or lorlatinib-treated mouse weights at start and end of treatment period. D. Kaplan Meier survival curve shows statistically significant difference in survivability of vehicle versus lorlatinib-treated U118MG tumor bearing mice. p<0.001 by Anova. E. Immunoblots from lysate prepared from mice treated as indicated. The illustration at the bottom indicates rough dissection lines used to harvest tissue to create normal brain (NB), and tumor brain (TB) as denoted in image. Lysates were interrogated with antibodies are shown in western blot panels, and dilutions described in Methods.