Figure 6. Impact of R191 on BCWM.1 xenografts in vivo.
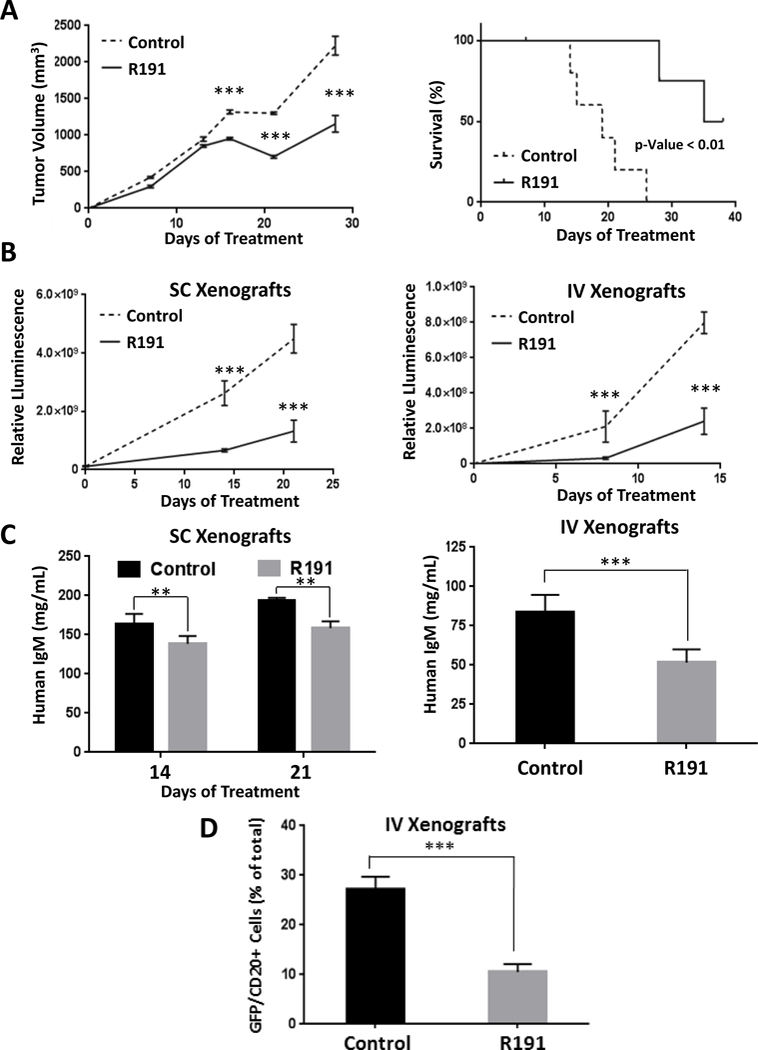
Mice were inoculated subcutaneously (SC) with 1×107 GFP+/luc+ BCWM.1 cells, or intravenously (IV) with 1×106 BCWM.1 GFP+/luc+ cells. They were then randomized and treated with intraperitoneal injections of R191 or vehicle (Control) with five mice per cohort. Growth of subcutaneous xenografts was measured by calipers and the mean values ± SD are shown (A), with “***” indicating p-values <0.0001 (left panel). The Kaplan-Meier method was also used to show survival of the xenograft-bearing mice (right panel). Survival was defined as the time until the tumors exceeded 15 mm in the largest dimension, at which point the animals were euthanized to avoid undue distress. In an independent set of experiments, tumor burden was measured by whole animal in vivo imaging in either a subcutaneous (SC; left panel) or systemic (IV; right panel) mouse model at the indicated time-points (B). The mean values ± SD are shown, and “***” indicates p-values <0.0001. As an added measure of disease burden, the serum levels of human IgM were determined by ELISA (C) in SC xenograft-bearing mice at treatment days 14 and 21 (left panel), and in IV xenograft-bearing mice at the end of the experiment (right panel). The proportion of GFP+/CD20+ cells in bone marrows of the systemic (IV) xenograft-bearing mice was evaluated by flow cytometry at the end of the experiment (3 weeks post-inoculation)(D). The mean values per group ± SD are shown, and “***” indicates p-values <0.0001.
