Figure 1.
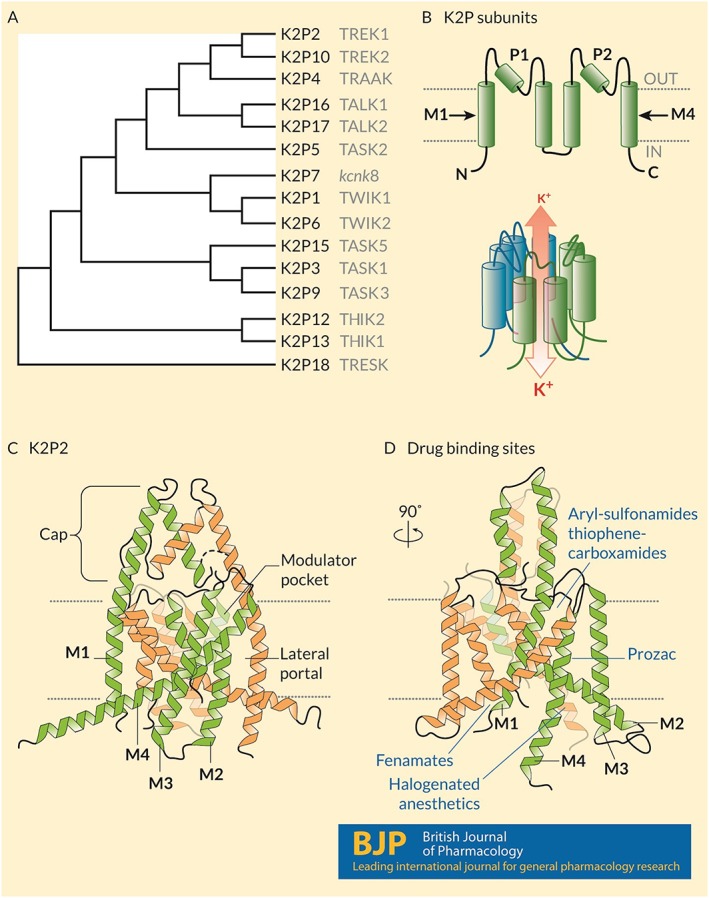
Structure and known drug interaction sites on K2P channels. (A) A phylogenetic tree calculated based on the primary sequences of the 15 K2P subunits expressed in humans. Common names appear in grey text. (B) (Upper) Humans express 15 K2P subunits with similar topologies: Intracellular N‐ and C‐termini, four‐transmembrane domains (M1–M4) and two re‐entrant pore loops (P1 and P2) that contribute to the K+ selective pore. (Lower) Two subunits come together as homodimers, and in some cases heterodimers, to form a K+ selective channel. Under physiological conditions, K+ ions move through the channel down their electrochemical gradients from the inside to the outside of the cell. (C) The crystal structure of mouse K2P2 (Lolicato et al., 2017) showing the architecture of the channel including the arrangement of the transmembrane domains, the extracellular cap above the plane of the membrane that bifurcates the K+ permeation pathway, the lateral portal and the modulator pocket. (D) A 90° rotation in the pose of K2P2 showing the location of four distinct drug binding sites: fenamates bind to the N‐terminus; aryl‐sulfonamides and thiophene‐carboxamides interact with the modulator pocket; fluoxetine (Prozac) interacts at the lateral port; and halogenated anaesthetics require the proximal C‐terminus of the channel and interact in a manner that involves Gαq proteins.
