ABSTRACT
Conventional prognostic scores for terminally ill cancer patients may have less objectivity because they include subjective or categorical variables that do not consider intensity or severity. The aim of this study was to identify prognostic factors for 30-day mortality from routine blood examination of terminally ill cancer patients. A total of 1308 study patients in a hospice setting were divided into investigation (n=761) and validation (n=547) groups. Twenty laboratory blood parameters were analyzed. Multivariate analysis revealed that ten variables (C-reactive protein ≥5.4 mg/dL, serum albumin <2.8 g/dL, blood urea nitrogen ≥21 mg/dL, white blood cell count ≥8.600 × 103/μL, eosinophil percentage <0.8%, neutrophil-to-lymphocyte ratio ≥11.1, hemoglobin level ≥ 13.2 g/dL, mean corpuscular volume ≥ 93.7 fl, red cell distribution width ≥ 16, and platelet count < 159 × 103/μL) were significant independent prognostic factors for 30-day survival. The laboratory prognostic score (LPS) was calculated by the sum of blood indices among the ten variables. The LPS showed acceptable accuracy for 30-day mortality in the investigation and validation groups. LPS 5 (including any five factors) predicted death within 30 days, with a sensitivity of 85%, a specificity of 55%, a positive predictive value of 72%, and a negative predictive value of 74%. The predictive value of LPS was comparable to those of conventional prognostic scores, which include signs and symptoms. The LPS can provide additional information to conventional prognostic scores.
Key Words: end-of-life care, palliative care, prognostic factors, prognosis, blood data
INTRODUCTION
Accurate prognostic information in palliative settings is necessary for patients to make decisions and set goals and priorities. Palliative care physicians should carefully provide patients and their families with the most accurate prognostic information in order to improve end-of-life care. A number of prognostic factors in terminal cancer patients, such as performance status, cancer anorexia, cachexia, dyspnea, and delirium have been identified, and several prognostic scores have been developed. These scores include the Palliative Prognostic (PaP) Score, Palliative Prognostic Index (PPI), Objective Prognostic Score (OPS), Japan Palliative Oncology Study Prognostic Index (JPOS-PI) and Prognosis Palliative Care Study (PiPS) predictor models.1-7) Because these conventional prognostic scores include subjective or categorical variables (e.g., clinical judgement, anorexia, or edema) for which the intensity or severity is less quantifiable, there can be limitations for more objective evaluation. The aim of this study was to identify, from routine laboratory blood examination, prognostic factors for 30-day mortality and to develop an objective additional prognostic model in terminally ill cancer patients.
PATIENTS AND METHODS
Study design and patient population
This retrospective study attempted to identify prognostic factors for 30-day mortality based on routine blood examination results for terminally ill cancer patients and to examine the internal validity of a laboratory test-based prognostic model. Between April 2006 and March 2014, a total of 1,766 terminally ill cancer patients with disseminated malignancy, who were no longer subject to specific anticancer therapy, were admitted to our hospice. Of these, 458 patients were excluded from this study due to a lack of comprehensive blood data. A total of 1308 patients were included in this study. Patients were divided into an investigation group (n=761), admitted to our hospice between April 2006 and March 2011, and a validation group (n=547), admitted to our hospice between April 2011 and March 2014. Data were obtained from the final blood test before hospice discharge in each patient and included C-reactive protein (CRP), serum albumin (Alb), total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, estimated glomerular filtration rate (eGFR), white blood cell (WBC) count, basophil percentage, eosinophil percentage (Eosino), neutrophil percentage, lymphocyte count, neutrophil-to-lymphocyte ratio (N/L), red blood cell (RBC) counts, hemoglobin (Hb) level, hematocrit (Ht), mean corpuscular volume (MCV), red cell distribution width (RDW), and platelet (Plt) count. The eGFR was calculated based on the serum creatinine level according to the equation recommended by the Japanese Society of Nephrology.8)
Our hospice care involves medical care, pain management, drug administration and infusion to reduce patients’ physical and psychological discomfort. In April 2013, the number of hospice beds was reduced from 25 to 20; however, there was no change in general care management or hospitalization criteria that included withdrawing life-sustaining treatment and cytoreductive therapy. There is no hospice standard for performing blood tests. Our hospice physicians usually perform blood test, if the blood test results can change the management of patient’s symptom.
The study protocol was approved by the Institutional Review Board of our hospital (approval reference number: 2016.082), which waived the requirement for informed consent owing to the retrospective nature of the study.
Statistical Analysis
Continuous variables, expressed as the median (interquartile range (IQR)), were compared using the Mann-Whitney U test. Differences in categorical data were compared using the chi-squared test. The Kaplan-Meier method was used to estimate survival curves, and the log-rank test was used to evaluate survival differences between groups. Follow-up information to hospice discharge was compiled for all patients, and survival was calculated from the day the final blood test was performed before hospice discharge until the date of hospice discharge. When the survival was calculated, patients who left the hospice alive were censored.
For univariate analyses in the investigation group, receiver operating characteristic (ROC) analysis was performed, and the area under each ROC curve (AUC) was calculated to assess the prognostic value for 30-day survival. In ROC analysis, the optimal cutoff values were determined to be the point where the vertical distance between the ROC curve and the diagonal line was maximal.
Multiple logistic regression analysis was performed using 30-day survival (yes/no) as the dependent variable.9) Variables with p<0.05 by univariate analysis were entered into the equation to identify significant independent prognostic factors of 30-day mortality, while AST and ALT were integrated to ALT, because they had a close relationship, and the AUC of ALT was larger than that of ALT. Similarly, red blood cell count, hemoglobin, and hematocrit were integrated to hemoglobin. In the validation group, the predictive factors identified from the investigation group were examined for validity. Statistical analyses were performed using JMP software (version 10.0 for Windows; SAS Institute Inc., Cary, NC, USA). Significance was set at a level of p <0.05.
RESULTS
Patient demographics
Patient demographics are presented in Table 1. The most common sites of primary malignancy were the respiratory, gastrointestinal, and hepatobiliary systems. Although there were statistically significant differences in albumin, ALT, WBC, lymphocyte count, MCV and Plt between the investigation and validation groups, there were no significant differences in age and sex. The mean duration between blood examination and hospice discharge in the investigation group was significantly longer than that in the validation group (24 days vs. 23 days, respectively); the 30-day survival of the investigation and validation group was 42.3% and 39.0%, respectively.
Table 1.
Patient demographics
| Investigation group
(n=761) |
Validation group
(n=547) |
p-value | |||
| Age | 73 (65–80) | 73 (65–79) | 0.5575 | ||
| Sex (Male : Female) | 479 : 282 | 342 : 205 | 0.8766 | ||
| Site of primary malignancy | 0.0484 | ||||
| Respiratory (including lung, pleura) | 298 | (39.2%) | 208 | (38.0%) | |
| Gastrointestinal | 225 | (29.6%) | 142 | (26.0%) | |
| Hepatobiliary | 105 | (13.8%) | 64 | (11.7%) | |
| Breast | 22 | (2.9%) | 19 | (3.5%) | |
| Bladder, kidney, urinary tracts | 21 | (2.8%) | 22 | (4.0%) | |
| Hematological | 20 | (2.6%) | 20 | (3.7%) | |
| Oral cavity | 16 | (2.1%) | 8 | (1.5%) | |
| Pharyngolarynx | 9 | (1.2%) | 11 | (2.0%) | |
| Female genital organs (including ovary, uterus) | 9 | (1.2%) | 21 | (3.8%) | |
| Head and neck (including thyroid, parotid gland) | 9 | (1.2%) | 6 | (1.1%) | |
| Male genital organs (including prostate) | 8 | (1.1%) | 11 | (2.0%) | |
| Skin | 6 | (0.8%) | 2 | (0.4%) | |
| Brain | 3 | (0.4%) | 1 | (0.2%) | |
| Others | 10 | (1.3%) | 12 | (2.2%) | |
| Blood examination data | |||||
| C-reactive protein (mg/dL) | 5.5 (1.9–10.6) | 5.2 (2.1–10.9) | 0.9725 | ||
| Albumin (g/dL) | 2.6 (2.2–3.1) | 2.8 (2.4–3.2) | 0.0019 | ||
| Total bilirubin (mg/dL) | 0.6 (0.4–1.1) | 0.6 (0.5–1.0) | 0.7574 | ||
| Aspartate aminotransferase (AST) (IU/L) | 30 (20–53) | 27 (19–51) | 0.2076 | ||
| Alanine aminotransferase (ALT) (IU/L) | 19 (13–37) | 17 (11–33) | 0.0095 | ||
| Blood urea nitrogen (BUN) (mg/dL) | 20 (14–30) | 20 (15–32) | 0.1522 | ||
| Creatinine | 0.74 (0.57–1.03) | 0.75 (0.55–1.12) | 0.6237 | ||
| Estimated glomerular filtration rate (eGFR) (ml/min/1.73 m2) | 71 (48–96) | 70 (46–96) | 0.5508 | ||
| White blood cell count (×103/μL) | 9.0 (6.4–13.1) | 8.4 (6.2–11.6) | 0.0251 | ||
| Basophil (%) | 0.3 (0.1–0.5) | 0.3 (0.1–0.6) | 0.0567 | ||
| Eosinophil (%) | 0.6 (0.2–1.6 | 0.4 (0.1–1.5) | 0.0333 | ||
| Neutrophil (%) | 80.7 (72.2–87.2) | 81.7 (73.5–88.3) | 0.1251 | ||
| Lymphocyte count (×103/μL) | 0.924 (0.591–1.344) | 0.804 (0.531–1.165) | 0.0003 | ||
| Neutrophil-to-lymphocyte ratio (N/L) | 7.6 (4.5–13.8) | 8.3 (4.8–15.2) | 0.0644 | ||
| Red blood cell count (×106/μL) | 3.33 (2.82–3.84) | 3.32 (2.71–3.81) | 0.4353 | ||
| Hemoglobin (g/dL) | 10.2 (8.8–11.7) | 10.2 (8.5–11.6) | 0.3058 | ||
| Hematocrit (%) | 30.3 (26.0–34.6) | 30.7 (25.8–34.9) | 0.8400 | ||
| Mean corpuscular volume (MCV) (fℓ) | 91.8 (87.2–96.8) | 92.8 (88.4–97.9) | 0.0102 | ||
| Red cell distribution width (RDW) | 16.6 (15.2–18.8) | 16.7 (15.0–18.8) | 0.9475 | ||
| Platelet count (×103/μL) | 257 (183–352) | 229 (158–308) | <0.0001 | ||
| Hospice discharge (Dead : Alive) | 740 : 21 | 515 : 32 | 0.0052 | ||
| Length of hospice stay (days) | 17 (7–38) | 19 (8–39) | 0.4105 | ||
| Duration between the last blood examination and hospice discharge (days) | 24 (11–48) | 23 (9–42) | 0.0242 | ||
| Median survival (days) after the last blood examination | 24 | 23 | 0.3792 | ||
| 30-day survival after the last blood examination | 42.3% | 39.0% | 0.3792 | ||
Continuous variables are expressed as the median (interquartile range), and categorical variables are described as numbers (percentages). Bold values indicate statistical significance (p<0.05).
Identification of significant independent prognostic factors for predicting 30-day survival
The AUC and optimal cutoff value for the 20 blood test data used to estimate 30-day survival are presented in Table 2. Univariate analyses of survival revealed that cutoff values of the 19 variables were statistically discriminative. Multivariate analysis showed that the following ten indices were significant independent prognostic factors for 30-day survival (Table 2): CRP ≥5.4 mg/dL, Alb <2.8 g/dL, BUN ≥21 mg/dL, WBC ≥8.600 × 103/μL, Eosino <0.8%, N/L ≥11.1, Hb ≥ 13.2 g/dL, MCV ≥ 93.7 fL, RDW ≥ 16, and Plt < 159 × 103/μL had odds ratios for 30-day mortality between 1.47 and 2.54.
Table 2.
Predictive value for 30-day survival of blood test data
| Blood test data | AUC | Optimal cut-off value | Univariate analysis | Multivariate analysis | |||||
| Number of
patients |
30-day
survival (%) |
p | Odds ratio
for 30-day mortality |
95%CI | p | ||||
| C-reactive protein | 0.6752 | 5.4 mg/dL | ≧5.4 | 385 | 29.4% | <0.0001 | 1.86 | 1.30 – 2.67 | 0.0007 |
| <5.4 | 376 | 55.5% | 1 | ||||||
| Albumin | 0.6275 | 2.8 g/dL | ≧2.8 | 294 | 56.0% | <0.0001 | 1 | 0.0006 | |
| <2.8 | 467 | 33.7% | 1.9 | 1.31 – 2.76 | |||||
| Total bilirubin | 0.5909 | 1.3 mg/dL | ≧1.3 | 151 | 23.8% | <0.0001 | 1.55 | 0.93 – 2.62 | 0.0953 |
| <1.3 | 610 | 46.8% | 1 | ||||||
|
Aspartate
aminotransferase (AST) |
0.5783 | 47 IU/L | ≧47 | 218 | 47.3% | <0.0001 | |||
| <47 | 243 | 31.2% | |||||||
| Alanine aminotransferase (ALT) | 0.6141 | 19 IU/L | ≧19 | 401 | 33.5% | <0.0001 | 1.44 | 0.99 – 2.29 | 0.0511 |
| <19 | 360 | 54.0% | 1 | ||||||
| Blood urea nitrogen (BUN) | 0.6782 | 21 mg/dL | ≧21 | 354 | 27.8% | <0.0001 | 1.98 | 1.33 – 2.95 | 0.0007 |
| <21 | 407 | 54.8% | 1 | ||||||
| Creatinine | 0.5481 | 1.09 mg/dL | ≧1.09 | 168 | 25.8% | <0.0001 | 1.80 | 0.86 – 3.79 | 0.1172 |
| <1.09 | 593 | 46.9% | 1 | ||||||
| Estimated glomerular filtration rate (eGFR) | 0.5577 | 45 ml/min/ 1.73 m2 | ≧45 | 596 | 46.6% | <0.0001 | 1 | 0.5568 | |
| <45 | 165 | 27.3% | 1.25 | 0.60 – 2.64 | |||||
| White blood cell count | 0.6589 | 8.600 × 103/μL | ≧8.600 | 417 | 30.2% | <0.0001 | 1.47 | 1.00 – 2.15 | 0.0495 |
| <8.600 | 344 | 57.0% | 1 | ||||||
| Basophil | 0.5940 | 0.1 % | ≧0.1 | 605 | 46.6% | <0.0001 | 1 | 0.4266 | |
| <0.1 | 156 | 25.4% | 1.22 | 0.75 – 2.00 | |||||
| Eosinophil | 0.6524 | 0.8 % | ≧0.8 | 304 | 59.0% | <0.0001 | 1 | 0.0349 | |
| <0.8 | 457 | 32.7% | 1.50 | 1.03 – 2.18 | |||||
| Neutrophil | 0.6551 | 83.7 % | ≧83.7 | 300 | 25.9% | <0.0001 | 1.02 | 0.59 – 1.78 | 0.9329 |
| <83.7 | 461 | 53.0% | 1 | ||||||
| Lymphocyte count | 0.4352 | 2.218 × 103/μL | ≧2.218 × 103 | 60 | 26.7% | 0.0019 | 1.84 | 0.92 – 3.81 | 0.0847 |
| <2.218 × 103 | 701 | 43.6% | 1 | ||||||
| Neutrophil-to-lymphocyte ratio (N/L) | 0.6684 | 11.1 | ≧11.1 | 257 | 22.5% | <0.0001 | 2.23 | 1.29 – 3.91 | 0.0043 |
| <11.1 | 504 | 54.3% | 1 | ||||||
| Red blood cell count | 0.5163 | 4.17 × 106/μL | ≧4.17 × 106 | 96 | 32.9% | 0.0309 | |||
| ≧4.17 × 106 | 665 | 43.6% | |||||||
| Hemoglobin | 0.5016 | 13.2 g/dL | ≧13.2 | 51 | 30.4% | 0.0101 | 2.54 | 1.34 –4.96 | 0.0041 |
| < 13.2 | 687 | 43.5% | |||||||
| Hematocrit | 0.5011 | 34.70% | ≧34.7 | 186 | 37.4% | 0.0749 | |||
| <34.7 | 575 | 43.8% | |||||||
| Mean corpuscular volume (MCV) | 0.5433 | 93.7 fL | ≧93.7 | 295 | 49.9% | <0.0001 | 1 | 0.0001 | |
| <93.7 | 466 | 37.5% | 2.07 | 1.43 – 3.02 | |||||
| Red cell distribution width (RDW) | 0.5871 | 16 | ≧16 | 474 | 35.4% | 0.0027 | 1.71 | 1.19 – 2.46 | 0.0034 |
| <16 | 287 | 53.4% | 1 | ||||||
| Platelet count | 0.5473 | 159 × 103/μL | ≧159 × 103 | 605 | 45.9% | <0.0001 | 1 | 0.0007 | |
| <159 × 103 | 156 | 28.2% | 2.19 | 1.39 – 3.50 | |||||
Bold values indicate statistical significance (p<0.05).
Prognostication by laboratory prognostic score (LPS)
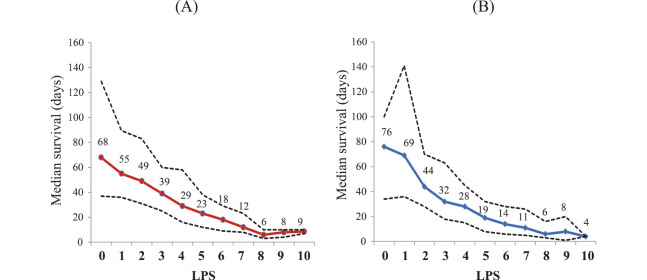
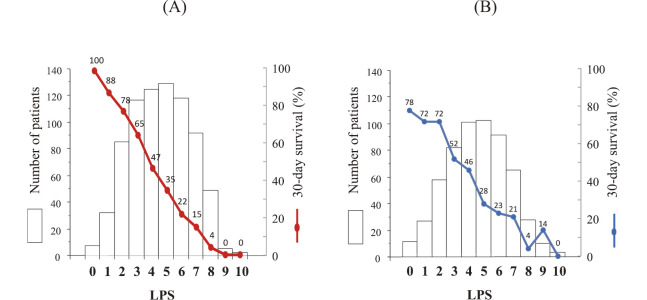
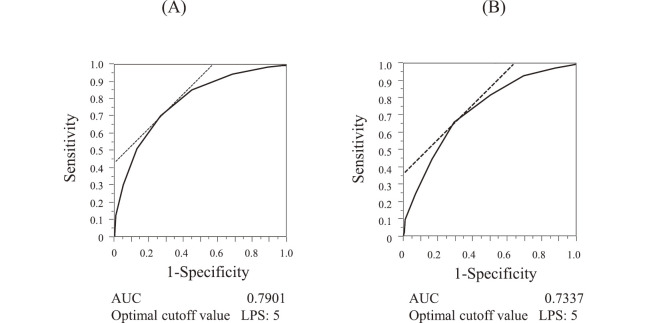
To develop a scoring system for 30-day mortality, laboratory prognostic score (LPS) was calculated by the sum of indices among the ten variables. In the investigation group, the median survival was decreased according to the LPS (Figure 1(A)). Figure 2(A) shows the number of patients with each LPS and 30-day survival, which was decreased from 100% to 0% according to the LPS between 0 and 10. The LPS ROC curve is shown in Figure 3(A); the AUC was 0.7901 at the optimal cutoff value of LPS 5. LPS 5 predicted death within 30 days, with a sensitivity of 85%, a specificity of 55%, a positive predictive value (PPV) of 72%, and a negative predictive value (NPV) of 74% (Table 3).
Fig. 1.
Median survival (solid line) and 25% and 75% percentiles (dotted lines) of patients with each laboratory prognostic score (LPS).
(A) Investigation group (n=761), (B) Validation group (n=547).
Fig. 2.
Number of patients with each laboratory prognostic score (LPS) (primary graph, presented as bars) and 30-day survival (secondary graph, presented as a line)
(A) Investigation group (n=761), (B) Validation group (n=547).
Fig. 3.
Receiver operating characteristic curve of laboratory prognostic score (LPS)
(A) Investigation group (n=761), (B) Validation group (n=547).
Table 3.
Thirty-day survival and predictive value for 30-day mortality classified by laboratory prognostic score (LPS) comprised of 10 blood indices (CRP ≥5.4 mg/dL, Alb <2.8 g/dL, BUN ≥21 mg/dL, WBC ≥8.600 × 103/μL, Eosino <0.8%, N/L ≥11.1, Hb ≥13.2 g/dL, MCV ≥93.7 fL, RDW ≥16, and Plt <159×103/μL).
| Investigation group | |||||||||||
| LPS | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Number of
patients |
7 | 32 | 85 | 117 | 125 | 129 | 118 | 92 | 49 | 5 | 2 |
| 30-day
survival (%) |
100% | 88% | 78% | 65% | 47% | 35% | 22% | 15% | 4% | 0% | 0% |
| Sensitivity
(%) |
100% | 100% | 100% | 99% | 94% | 85% | 70% | 51% | 30% | 12% | 1% |
| Specificity
(%) |
0% | 2% | 2% | 11% | 31% | 55% | 73% | 87% | 95% | 99% | 100% |
| Positive
predictive value (%) |
57% | 58% | 58% | 60% | 65% | 72% | 78% | 84% | 89% | 96% | 100% |
| Negative
predictive value (%) |
– | 100% | 78% | 85% | 81% | 74% | 65% | 57% | 50% | 46% | 43% |
| Validation group | |||||||||||
| LPS | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Number of
patients |
9 | 24 | 55 | 79 | 98 | 99 | 88 | 62 | 25 | 7 | 1 |
| 30-day
survival (%) |
78% | 72% | 72% | 52% | 46% | 28% | 23% | 21% | 4% | 14% | 0% |
| Sensitivity
(%) |
100% | 99% | 99% | 98% | 93% | 82% | 66% | 44% | 24% | 9% | 2% |
| Specificity
(%) |
0% | 3% | 3% | 12% | 30% | 50% | 71% | 83% | 93% | 99% | 100% |
| Positive
predictive value (%) |
60% | 61% | 61% | 63% | 67% | 71% | 77% | 80% | 84% | 94% | 86% |
| Negative
predictive value (%) |
– | 78% | 70% | 76% | 74% | 64% | 58% | 50% | 45% | 42% | 40% |
Validation of LPS for predicting 30-day mortality
In the validation group, the median survival was decreased according to the LPS (Figure 1(B)). Figure 2(B) shows the number of patients with each LPS and 30-day survival, which was decreased from 78% to 0% according to the LPS between 0 and 10. The LPS ROC curve is shown in Figure 3(B); the AUC was 0.7337 at the optimal cutoff value of LPS 5. LPS 5 predicted death within 30 days, with a sensitivity of 82%, a specificity of 50%, a PPV of 71%, and a NPV of 64% (Table 3).
DISCUSSION
The present study revealed that ten blood indices were independent prognostic factors for 30-day mortality of terminally ill cancer patients. The LPS comprising these ten indices showed acceptable accuracy, which was comparable to those of the validation group.
Conventional prognostic scores previously reported in the literature are based on physical signs, symptoms, and psychological factors with/without data from laboratory blood tests.1-7,10-11) Those prognostic scores are universally available and useful for terminal care of cancer patients; however, their potential limitation is the inclusion of subjective or categorical variables such as physician’s judgment (i.e., clinical prediction of survival), dyspnea, anorexia, edema, pleural effusion, or consciousness, for which the intensity or severity is less quantifiable; and consequently, there can be limitations for more objective evaluation.12) Table 4 shows reported predictive values of several prognostic scores for terminally ill cancer patients.4-5,13-14) The predictive value of the LPS was comparable to those of conventional prognostic scores, although the median survivals were different among the studies (22–27 days).
Table 4.
Comparison of the predictive values among conventional prognostic scores and laboratory prognostic score
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Predicting Model | Objective Prognostic Score (OPS) | Palliative Prognostic Score (PaP Score) | Palliative Prognostic Index (PPI) | Laboratory Prognostic Score (LPS) | ||
| Year | 2010 | 2012 | 1999 | 2012 | 2017 | |
| Author | Suh SY | Maltoni M | Morita T | Maltoni M | Yamada T | Kawai N |
| Country | South Korea | Italy | Japan | Italy | Japan | Japan |
| Number of patients | 185 | 549 | 150 | 549 | 892 | 761 |
| Setting | inpatient | in hospice | in hospice | in hospice | in palliative care unit | in hospice |
| Median survival | 26 days | 22 days | 27 days | 22 days | 25 days | 24 days |
| 30-day survival | nd | nd | nd | nd | nd | 42% |
| 3-week survival | nd | nd | 63% | nd | nd | 57% |
| Score range | 0–8 | 0–17.5 | 0–15.0 | 0–15.0 | 0–15.0 | 0–10 |
| Mortality prediction | 3 weeks | 30 days | 3 weeks | 30 days | 3 weeks | 30 days |
| Cutoff value | OPS: 3 | PaP Score: 5 | PPI: 6.0 | PPI: 4.0 | PPI: 6.0 | LPS: 5 |
| Sensitivity | 75 % | 92 % | 75 % | 85 % | 71 % | 85 % |
| Specificity | 77 % | 58 % | 84 % | 54 % | 67 % | 55 % |
| Positive Predictive Value | 42 % | 76 % | 73 % | 73 % | 63 % | 72 % |
| Negative Predictive Value | 79 % | 82 % | 85 % | 70 % | 75 % | 74 % |
nd: not described
Many studies have found several biomarkers to have prognostic value for terminally ill cancer patients: lymphocyte count, WBC, lactate dehydrogenase, BUN, Hb, CRP, Alb, and creatinine.2,5-6,15-18) The current study implies that the sum of blood indices of the ten indices (CRP ≥5.4 mg/dL, Alb <2.8 g/dL, BUN ≥21 mg/dL, WBC ≥8.600 × 103/μL, Eosino <0.8%, N/L ≥11.1, Hb ≥ 13.2 g/dL, MCV ≥ 93.7 fL, RDW ≥ 16, and Plt < 159×103/μL) has an acceptable prognostic value. An elevated CRP, WBC and N/L suggest inflammation or overproduction of cytokine produced by malignant tumor, while a decreased Alb implies malnutrition. An increased BUN suggests dehydration, gastrointestinal bleeding, or impaired renal function. Decreased immune status is suggested by an elevated N/L. A decreased platelet count implies bone marrow suppression or bleeding tendency. An elevated Hb suggests dehydration.
To the best of our knowledge, this is the first study showing a significant prognostic value of eosinophil percentage, MCV, and RDW in terminally ill cancer patients. Eosinophils are a component of the innate immune system and have a variety of functions.19) Several studies have reported a lower eosinophil count as a worse prognostic marker in patients with acute heart failure, chronic obstructive pulmonary disease, critical medical illness, and bacteremia.20-23) The MCV indicates the volume of RBC and is frequently used for the diagnosis of megaloblastic or iron-deficiency anemia. A high MCV was associated with worse outcomes in patients with coronary artery disease and renal failure.24-25) The RDW is a measure of the range of variation of RBC volume and has traditionally been used to differentiate various types of anemia.26) The evidence associating RDW with a higher risk of mortality has been reported in patients with coronary disease, heart failure, acute cerebral infarction, and septic shock.27-30)
To improve the discriminative ability of LPS, we developed a scoring system in which each one of ten categories was assigned various points based on the logarithm of odds ratio. The AUC of this model was 0.7528, which was lower than the AUC of the original LPS model developed from the sum of indices. We, therefore, adopted the original LPS model.
We acknowledge that our study has several limitations. First, this study was retrospective and was conducted at a single hospice; thus, the results could not be extrapolated to other palliative care settings (e.g., hospital palliative care, home palliative care, and patients undergoing antiblastic therapy). Second, venipuncture is necessary for LPS determination. It may not be easy for physicians to perform invasive procedures on frail patients. Third, the timing of blood examination was not specifically planned for prognostication but mostly for symptom management, and the last day at which blood samples were collected was chosen among several days for analyzing 30-mortality. Scheduled blood sampling should have been performed for accurate prognostic estimation; however, this could not be done because of the retrospective nature of the study. Fourth, this study included patients with hematological malignancy. Such patients often have blood disorder even in the early phase of disease; therefore, LPS may be less prognostic for patients with hematological malignancy. Fifth, this study did not compare the prognostic values between conventional prognostic scores and LPS in each patient. The comparison may reveal the significance of LPS; however, it was not performed due to the limited number of patients who were evaluated by conventional prognostic scores. Sixth, although this study revealed ten indices (CRP ≥5.4 mg/dL, Alb <2.8 g/dL, BUN ≥21 mg/dL, WBC ≥8.600 × 103/μL, Eosino <0.8%, N/L ≥11.1, Hb ≥ 13.2 g/dL, MCV ≥ 93.7 fL, RDW ≥ 16, and Plt < 159 × 103/μL) that have an acceptable prognostic value in terminally ill cancer patients, we could not provide sufficient meaning in some indices.
The strength of this study was the large number of patients and comprehensive analysis of routine blood tests. The predictive value of LPS was comparable to that of conventional prognostic scores. Although the estimation of 30-day mortality by LPS requires venipuncture, it was objective and easily understandable. The LPS can be used in combination with physical signs and symptoms to improve prognostication. Future studies should focus on validating our results and estimating more accurately the short-term mortality of terminally ill cancer patients.
ACKNOWLEDGEMENTS
We acknowledge the assistance of Hideki Kato, who collected the blood data.
CONFLICTS OF INTEREST
Natsuko Kawai and Norihiro Yuasa do not have any conflict of interest to disclose.
REFERENCES
- 1). Pirovano M, Maltoni M, Nanni O, Marinari M, Indelli M, Zaninetta G, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage, 1999; 17: 231–239. [DOI] [PubMed]
- 2).Maltoni M, Nanni O, Pirovano M, Scarpi E, Indelli M, Martini C, et al. Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian Multicenter study Group on pa11iative care. J Pain Symptom Manage, 1999; 17: 240–247. [DOI] [PubMed]
- 3).Scarpi E, Maltoni M, Miceli R, Mariani L, Caraceni A, Amadori D, et al. Survival prediction for terminally ill cancer patients: revision of the palliative prognostic score with incorporation of delirium. Oncologist, 2011; 16: 1793–1799. [DOI] [PMC free article] [PubMed]
- 4).Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer, 1999; 7: 128–133. [DOI] [PubMed]
- 5).Suh SY, Choi YS, Shim JY, Kim YS, Yeom CH, Kim D, et al. Construction of a new, objective prognostic score for terminally ill cancer patients: a multicenter study. Support Care Cancer, 2010; 18: 151–157. [DOI] [PubMed]
- 6).Hyodo I, Morita T, Adachi I, Shima Y, Yoshizawa A, Hiraga K. Development of a predicting tool for survival of terminally ill cancer patients. Jpn J Clin Oncol, 2010; 40: 442–448. [DOI] [PubMed]
- 7).Gwilliam B, Keeley V, Todd C, Gittins M, Roberts C, Kelly L, et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ, 2011 25; 343: d4920. [DOI] [PMC free article] [PubMed]
- 8).Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009; 53: 982–992. [DOI] [PubMed]
- 9).Katz MH. Multivariate analysis: a practical guide for clinicians. 2008, Cambridge University Press, Cambridge.
- 10).Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med, 1988; 148: 1586–1591. [PubMed]
- 11).Bruera E, Miller MJ, Kuehn N, MacEachern T, Hanson J. Estimate of survival of patients admitted to a palliative care unit: a prospective study. J Pain Symptom Manage, 1992; 7: 82–86. [DOI] [PubMed]
- 12).Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ, 2003; 327: 195–198. [DOI] [PMC free article] [PubMed]
- 13).Maltoni M, Scarpi E, Pittureri C, Martini F, Montanari L, Amaducci E. Prospective comparison of prognostic scores in palliative care cancer populations. Oncologist, 2012; 17: 446–454. [DOI] [PMC free article] [PubMed]
- 14).Yamada T, Morita T, Maeda I, Inoue S, Ikenaga M, Matsumoto Y, et al. A prospective, multicenter cohort study to validate a simple performance status–based survival prediction system for oncologists. Cancer, 2017; 123: 1442–1452. [DOI] [PubMed]
- 15).Kikuchi N, Ohmori K, Kuriyama S, Shimada A, Nakaho T, Yamamuro M, et al. Survival prediction of patients with advanced cancer: the predictive accuracy of the model based on biological markers. J Pain Symptom Manage, 2007; 34: 600–606. [DOI] [PubMed]
- 16).Kao YH, Chen CN, Chiang JK, Chen SS, Huang WW. Predicting factors in the last week of survival in elderly patients with terminal cancer: a prospective study in southern Taiwan. J Formos Med Assoc, 2009; 108: 231–239. [DOI] [PubMed]
- 17).Chiang JK, Lai NS, Wang MH, Chen SC, Kao YH. A proposed prognostic 7-day survival formula for patients with terminal cancer. BMC Public Health, 2009; 29: 365. [DOI] [PMC free article] [PubMed]
- 18).Miura T, Matsumoto Y, Hama T, Amano K, Tei Y, Kikuchi A, et al. Glasgow prognostic score predicts prognosis for cancer patients in palliative settings: a subanalysis of the Japan-prognostic assessment tools validation (J-ProVal) study. Support Care Cancer, 2015; 23: 3149–3156. [DOI] [PubMed]
- 19).Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol, 2012; 5: 157–176. [DOI] [PMC free article] [PubMed]
- 20).Cikrikcioglu MA, Soysal P, Dikerdem D, Cakirca M, Kazancioglu R, Yolbas S, et al. Absolute blood eosinophil count and 1-year mortality risk following hospitalization with acute heart failure. Eur J Emerg Med, 2012; 19: 257–263. [DOI] [PubMed]
- 21).Holland M, Alkhalil M, Chandromouli S, Janjua A, Babores M. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology, 2010; 15: 165–167. [DOI] [PubMed]
- 22).Abidi K, Belayachi J, Derras Y, Khayari ME, Dendane T, Madani N, et al. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med, 2011; 37: 1136–1142. [DOI] [PubMed]
- 23).Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One, 2012; 7: e42860. Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0042860 Accessed on July 29, 2018. [DOI] [PMC free article] [PubMed]
- 24).Myojo M, Iwata H, Kohro T, Sato H, Kiyosue A, Ando J, et al. Prognostic implication of macrocytosis on adverse outcomes after coronary intervention. Atherosclerosis, 2012; 221: 148–153. [DOI] [PubMed]
- 25).Tennankore KK, Soroka SD, West KA, Kiberd BA. Macrocytosis may be associated with mortality in chronic hemodialysis patients: a prospective study. BMC Nephrol, 2011; 12: 19. [DOI] [PMC free article] [PubMed]
- 26).Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc, 2005; 80: 923–936. [DOI] [PMC free article] [PubMed]
- 27).Lappé JM, Horne BD, Shah SH, May HT, Muhlestein JB, Lappé DL, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta, 2011; 412: 2094–2099. [DOI] [PubMed]
- 28).Kim J, Kim YD, Song TJ, Park JH, Lee HS, Nam CM, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost, 2012; 108: 349–356. [DOI] [PubMed]
- 29).Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med, 2013; 28: 307–313. [DOI] [PubMed]
- 30).Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J, 2009; 158: 659–666. [DOI] [PubMed]