Abstract
Respiratory virus infections account for a significant proportion of acute admissions to the paediatric intensive care unit (PICU). Recent studies have shown that rhinoviruses (RV) are the most frequent virus detected in severe cases of acute respiratory illnesses (ARI) admitted to a PICU. The aim of this study was to determine the prevalence of different viruses, in particular RV species, in children with ARI admitted to a tertiary PICU. Nasopharyngeal aspirates (NPA) from 229 children admitted to PICU with an ARI were analysed. RV was the most common virus detected, being present in 94 (41.0%) of samples examined, followed by respiratory syncytial virus (RSV) which was identified in 50 (21.8%) samples. A subsection analysis of cases with residual sample available of sufficient quality to allow for RV species typing showed that overall, the percentage of PICU admissions for each RV species was 22.3% for RV-C, 17.5% for RV-A and 1.7% for RV-B. This study demonstrated that RV is the most frequent virus identified in children admitted to a tertiary PICU with an ARI and RV-C is the most common RV species detected. Importantly, in the children admitted to PICU with an ARI, RV-C was by itself as common a pathogen as RSV.
Keywords: Rhinovirus, respiratory illness, children, PICU
Background
Acute respiratory illnesses (ARIs) account for 10–15% of all admissions to the paediatric intensive care unit (PICU) [1]. Historically, RSV was reported to be the most common viral pathogen resulting in admission to PICU [2]. Recent studies, using more sensitive molecular techniques, have shown that rhinoviruses (RV) may be the most frequent virus detected in respiratory admissions to a PICU [3–5].
We have previously reported that the RV-C species is associated with more frequent respiratory admissions [6] [7]. There has been no study reported to date examining the prevalence of each RV species in children admitted with an ARI to PICU.
The aim of this study was to determine the prevalence of different viruses, in particular RV species, in children admitted to a PICU with an ARI.
Methods
Patient information was obtained retrospectively from the hospital computer database as part of an audit on all children admitted with an ARI to PICU at Princess Margaret Hospital (PMH), Perth, Australia between March 2009 and July 2011.
We compared these data collected with that from a concurrent prospective study running at PMH examining the mechanisms of acute viral respiratory infections in children (MAVRIC) [7]. The MAVRIC study enrolled children presenting to the emergency department (ED) with acute asthma, bronchiolitis and pneumonia. We compared our PICU cohort with all cases with the above ARIs not requiring PICU admission that were recruited to the MAVRIC study over the same timeframe.
Detection of common respiratory viruses from nasopharyngeal aspirate (NPA) samples were completed as previously described [6, 8–10].
Results
From March 2009 to July 2011, 260 children were admitted to PICU with an ARI in total. Of this cohort, 229 (88.1%) had an NPA performed. The mean age was 2.8± standard deviation (SD) of 4.0 (range 0–16) years and the most common respiratory diagnoses were acute asthma (n=63, 27.5%), bronchiolitis (n=67, 29.3%) and pneumonia (n=44, 19.2%).
RV was the most frequent virus detected; being present in 94 (41.0%) of the NPA samples. RSV was identified in 50 (21.8%) samples.
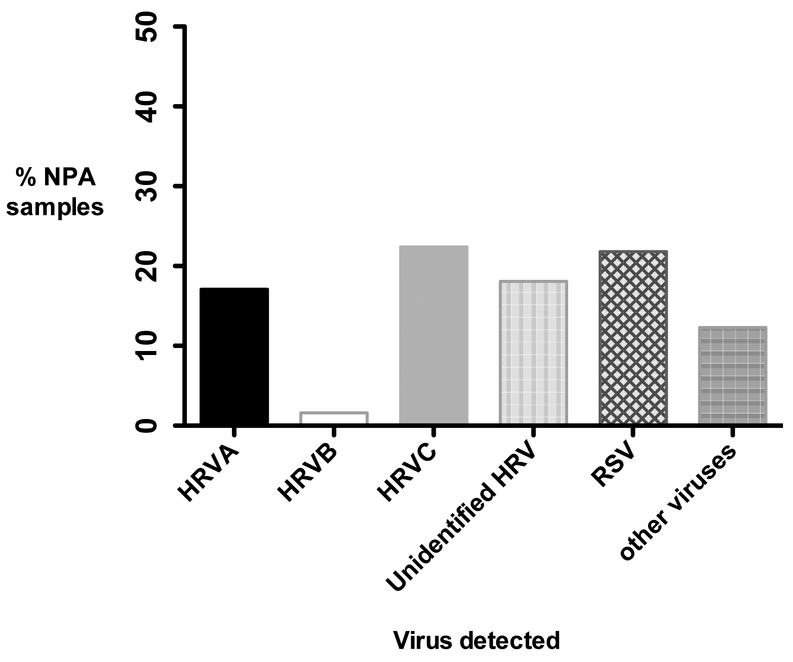
Based on the analysis of prevalence of RV species, RV-C was the most common RV species detected, being present in 51 (22.3%) of the 229 samples (Figure 1). RV-A species was found in 40 (17.5%) and RV-B in 4 (1.7%) of the overall PICU cohort.
Figure 1.
Of the overall 260 patients admitted to PICU with an ARI, 31 (11.9%) did not have an NPA taken. The demographics of these patients were compared with those PICU respiratory admissions that had an NPA taken. The children who did not have an NPA performed were older (6.41±5.34 versus 2.8±4.0, p<0.001) and more likely to have a diagnosis of pneumonia (35.5% versus 19.2%, p=0.01).
Over the duration of this study, 181 ED ARI cases were recruited to the MAVRIC study and these subjects were compared with the cohort admitted to PICU. Patients admitted to PICU were more likely to stay in hospital longer (23.7±54.5 versus 1.4±1.39 days, p<0.001) and have a co-morbidity (n=88, 38.4% versus n=0; p<0.001) compared with ED ARI cases admitted to the ward.
Subjects admitted to PICU versus ED ARI cases were less likely to have a diagnosis of asthma (27.5% versus 66.8%, p<0.001, respectively) but more likely to have a diagnosis of pneumonia (19.6% versus 5.6%, p<0.001), respectively. RVC was the most common RV species detected in both sets of hospital cases. There was no statistical difference identified between the two groups with any particular virus.
Of the 229 children admitted to PICU who had NPA, 24 (10.5%) had a co-infection with another virus. Comparing clinical outcomes of the children who had a co-infection with the children with one virus detected, there was no statistically significant difference between the two groups.
Discussion
This study shows that RV is the most common virus identified in children admitted to a PICU with an ARI and RV-C is the most frequent RV species detected in children positive for RV. Importantly, our data show that RV-C is a common viral pathogen detected in PICU ARI admissions and as common, or perhaps even more common than RSV.
This is first study to systematically examine relationships between respiratory viruses and different RV species in children admitted to a PICU with an ARI and to our knowledge is the largest study in a paediatric PICU population using RT-PCR RV molecular typing methods. Previous studies either did not include RV typing and used older virological detection methods [2] or were too small to report specifically on the prevalence of RV species [3, 4].
Our research group has previously demonstrated that RV-C-related wheezing episodes in preschool children are associated with an increased risk of previous and subsequent respiratory hospital admissions compared with other RV species [7]. Recent experimental evidence established that RV-C species has the ability to grow equally well at both 34°C and 37°C [11] which helps to explain why RV-C species are frequently found in severe lower respiratory illnesses in children.
Major strengths in this study include the use of up-to-date, highly sensitive PCR techniques and that it included a number of different respiratory diagnoses over different seasons. The findings in our study are strengthened by the inclusion of a control group. Although there were some differences in the clinial demographics between the two groups reported, the MAVRIC ARI cases provide corroborating evidence on the frequency of different viruses in children admitted to PICU with an ARI.
In this study, viral detection data was not available on 31 (11.9%) of the overall PICU respiratory admissions. These children were older and more likely to have a diagnosis of pneumonia. NPAs are not routinely performed on older children given the level of discomfort associated with the procedure. Both the PICU and ED ARI cohorts where an NPA was performed were mainly younger pre-school children admitted with either bronchiolitis or asthma. Previous studies have demonstrated a similar frequency and distribution of RV infections in preschool children with wheezing disorders verifying our findings [7, 12–15]. A subsection analysis was required to determine RV species as 91 (40%) of the collected NPAs were no longer available for RV typing. Given the size of this subanalysis and that the availability of these specimens was random, the data reported could be expected to represent the profile of the distribution of different RV species in children admitted to PICU with an ARI.
In conclusion, this is first report examining the role of different RV species in ARIs in children admitted to a teritary PICU. RV was the most common virus detected and RV-C was the most prevalent RV species detected. RV-C was also the most common RV species detected in children admitted to PICU with a diagnosis of either acute asthma or bronchiolitis. Importantly, RV-C was as commonly detected in PICU admissions as RSV.
Table 1:
Patient demographics of PICU cases compared with ED ARI cases
| Demographics | PICU cases n=229 | EDARI cases n=181 |
|---|---|---|
| Age (years), range | 2.8 ± 4.0, 0–16 | 3.2 ± 2.7, 0–15 |
| Male (%) | 141 (61.6) | 113 (62.5) |
| LOS in PICU (hours) | 80.7 ± 145.8 | - |
| LOS in hospital (days) | 23.7 ± 54.5 | 1.4 ± 1.39* |
| Mechanical ventilation required | 79(34.5) | - |
| Associated co-morbidity | 88 (38.4) | 0 (0)* |
| Admission diagnosis | ||
| Asthma | 63 (27.5) | 121 (66.8)* |
| Bronchiolitis | 67 (29.3) | 32 (17.7) |
| Pneumonia | 44 (19.2) | 10 (5.6)* |
| Chronic lung disease | 16 (7.0) | 0 (0)* |
| LRI other | 22 (9.6) | 18 (9.9) |
| Respiratory failure | 10 (4.4) | 0 (0)* |
| Other respiratory admission diagnoses | 7 (3.1) | 0 (0)* |
| Seasons | ||
| Spring | 61 (26.6) | 36 (19.9) |
| Summer | 25 (10.9) | 9 (5.0) |
| Autumn | 53 (23.1) | 48 (26.5) |
| Winter | 90 (39.3) | 88 (48.6) |
Data above is presented as n (%) and mean ± SD unless otherwise stated. LOS = Length of stay; LRI = Lower respiratory illness; PICU = Paediatric intensive care unit;
p<0.05 for PICU cases vs. ED ARI acute cases
Table 2:
Distibution of viruses between the PICU and ED ARI cases
| PICU cases n=229 | ED ARI cases n=181 | |
|---|---|---|
| RV | 94/229 (41.0) | 122/176 (69.3) |
| RVA* | 40/229 (17.5) | 40/181 (22.1) |
| RVB* | 4/229 (1.7) | 3/181 (1.7) |
| RVC* | 51/229 (22.3) | 80/181 (44.2) |
| RSV | 50/229 (21.8) | 38/179 (21.2) |
| Adenovirus | 2/229 (0.9) | 2/178 (3.8) |
| Influenza | 6/229 (2.6) | 0/178 (0) |
| Parainfluenza | 2/229 (0.9) | 9/178 (5.1) |
| Human metapneumovirus | 6/229 (2.6) | 7/178 (3.9) |
| Enterovirus | 2/229 (0.9) | 4/178 (2.2) |
| Other virus | 6/229 (2.6) | 6/178 (3.3) |
| Combination | 11/229 (4.8) | 25/178 (14.0) |
| No virus | 61/229 (26.6) | 21/178 (11.8) |
Data above is presented as n (%)
HRV species prevalence data is presented here
Highlights.
This is first study to systematically examine relationships between respiratory viruses and rhinovirus species in children admitted to a PICU with a respiratory illness using up to date PCR techniques
Acknowledgments
Funding
This study was supported by National Health and Medical Research Council (NHMRC) funding.
References
- 1.Purcell K and Fergie J, Driscoll Children’s Hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatr Infect Dis J, 2004. 23(5): p. 418–23. [DOI] [PubMed] [Google Scholar]
- 2.Straliotto SM, et al. , Respiratory viruses in the pediatric intensive care unit: prevalence and clinical aspects. Mem Inst Oswaldo Cruz, 2004. 99(8): p. 883–7. [DOI] [PubMed] [Google Scholar]
- 3.Louie JK, et al. , Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J, 2009. 28(4): p. 337–9. [DOI] [PubMed] [Google Scholar]
- 4.Aramburo A, et al. , Role of real-time reverse transcription polymerase chain reaction for detection of respiratory viruses in critically ill children with respiratory disease: Is it time for a change in algorithm? Pediatr Crit Care Med, 2011. 12(4): p. e160–5. [DOI] [PubMed] [Google Scholar]
- 5.Lonngren C, et al. , North-South divide: distribution and outcome of respiratory viral infections in paediatric intensive care units in Cape Town (South Africa) and Nottingham (United Kingdom). J Paediatr Child Health, 2014. 50(3): p. 208–15. [DOI] [PubMed] [Google Scholar]
- 6.Bizzintino J, et al. , Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J, 2011. 37(5): p. 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox DW, et al. , Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med, 2013. 188(11): p. 1358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WM, et al. , High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol, 2007. 45(8): p. 2626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WM, et al. , A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One, 2007. 2(10): p. e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochkov YA, et al. , Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol, 2014. 52(7): p. 2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashraf S, et al. , Biological characteristics and propagation of human rhinovirus-C in differentiated sinus epithelial cells. Virology, 2013. 436(1): p. 143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller EK, et al. , A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol, 2009. 123(1): p. 98–104 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller EK, et al. , Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol, 2009. 46(1): p. 85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwane MK, et al. , Human Rhinovirus Species Associated With Hospitalizations for Acute Respiratory Illness in Young US Children. J Infect Dis, 2011. 204(11): p. 1702–10. [DOI] [PubMed] [Google Scholar]
- 15.Khetsuriani N, et al. , Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis, 2008. 14(11): p. 1793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]