Abstract
Sinus congestion resultant of allergic rhinosinusitis is associated with development and worsening of asthma and can result in difficulty breathing, headaches, and missed days of school and work. Quantification of sinus congestion is important in the understanding of allergic rhinosinusitis and the development of new drugs for its treatment. Noninvasive microcomputed tomography (micro-CT) was investigated in a guinea pig model of allergic rhinosinusitis to determine its utility to determine accurately the degree of sinus congestion and resolution with anti-inflammatory drug administration. Three-dimensional sinus air-space volume, two-dimensional sinus width, sinus image air-space area, and sinus image sinus perimeter were measured in guinea pigs administered ragweed pollen (RWP), intranasally (i.n.), followed by administration of fluticasone, i.n. To determine their relative accuracy in assessing sinus congestion, the micro-CT image results were compared with the “gold-standard” method of sinus fluid fill-volume (SFFV) measurements. As measured by SFFV method, RWP increased sinus congestion in a RWP concentration-dependent fashion, approaching near-total sinus blockage with concentrations ≥22 µg of RWP. At this level of congestion, fluticasone (25–100 µg) progressively decreased sinus congestion in a concentration-dependent fashion. The noninvasive micro-CT methods were found to accurately determine the amount of sinus congestion and resolution, with patterns of increases and decreases of congestion that were nearly identical to the SFFV method. We conclude that noninvasive micro-CT measurements of allergic sinus congestion can be useful as an investigative tool in the assessment of congestion intensity and the development of new drug therapies for its treatment.
NEW & NOTEWORTHY Allergic rhinosinusitis afflicts significant portions of the world population, resulting in loss of work productivity and decreased quality of life. Thus the development of methodological approaches, which incorporate accurate and reproducible noninvasive assessments of sinus congestion, are desirable. Microcomputed tomography of the guinea pig sinuses offers a noninvasive evaluation tool in an animal model of IgE-dependent allergy similar to that in humans, with potential relevance toward development of therapeutics for human sinus diseases.
Keywords: allergic sinus inflammation, guinea pig, micro-CT, ragweed pollen, rhinosinusitis
INTRODUCTION
Allergic rhinosinusitis is one of the most common diseases of inflammation of the upper airways in humans; oftentimes, allergic rhinosinusitis also coexists with, and exacerbates, asthma (5, 14, 30). Clinically, allergic rhinosinusitis is characterized by early-phase symptoms such as sneezing, rhinorrhea, and lacrimation, whereas late-phase symptoms include sinus congestion and drainage, which can lead to headache, coughing, sore throat, and significant breathing difficulties (16, 50), with the increased airflow resistance due to reduction in sinus airway caliber. Specifically, histamine, stored primarily in mast cells, is known to play a primary role in the late-phase inflammatory reactions of allergic rhinosinusitis, which are IgE-dependent, and associated with asthma (1, 46, 47, 51). Typical therapeutic treatments for allergic rhinosinusitis include antihistamines, inhaled nasal corticosteroids, and, in some cases, regular sinus washings (4, 24, 41). Although effective to varying degrees, there are issues related to side effect profiles, inconvenience, and patient compliance that can limit the effectiveness of those therapeutic approaches. Thus exploration of new sinus congestion assessment techniques and potential therapeutic agents in the study of allergic rhinosinusitis are desirable (22, 23).
Quantitative assessment of sinus congestion in humans can be challenging due to the structure of the nasal sinuses and the invasiveness required by a number of methods in obtaining reliable measurements (e.g., nasal endoscopy and biopsy). The development of a reliable animal model with translational relevance to human allergic rhinosinusitis is desirable to facilitate study of new therapeutic agents for potential development in humans. Although mouse models of allergic rhinosinusitis have been beneficial, they are associated with limitations of size and, most importantly, have serotonin as the major preformed mediator released from mast cells associated with the IgE-dependent allergic response (25, 26, 34). In contrast, guinea pigs, which also have been used for many years in studies of allergic airway inflammation (3, 6, 42), can represent a more relevant model of human allergic rhinosinusitis, due to having a primarily mast cell histamine-driven IgE allergic response (31, 49).
Accordingly, the objective of the present study was to compare 2 noninvasive computed tomography image-based approaches of measuring sinus congestion (2- and 3-dimensional microcomputed axial tomography assessments) with a method that utilizes direct quantitation of sinus congestion based on a sinus fluid fill-volume (SFFV) method adapted from prior published studies using fluid displacement in the guinea pig (44). First, we quantified the magnitude and drug-dependent resolution of the sinus allergic response to ragweed pollen (RWP) extract with the SFFV method. These results were then compared with 2-dimensional (2-D) and 3-dimensional (3-D) microcomputed axial tomography (micro-CT) methods to determine the consistency of allergic and resolution effects and the comparability of noninvasive imaging methods with the direct SFFV assessment method. Our findings indicated that the guinea pig was a sensitive model of allergic rhinosinusitis and anti-inflammatory treatment and that noninvasive digital imaging methods could be utilized reliably and with accuracy of response characterization that was similar to the direct quantitation of allergic rhinosinusitis using the SFFV method.
MATERIALS AND METHODS
Animals.
Male Hartley guinea pigs (9–12 wk old; weight 600–800 g at time of experiments) were purchased from Charles River and housed in high-efficiency particulate-filtered air cages with free access to food and water. A total of 42 male guinea pigs were used in the experiments, including 16 guinea pigs for RWP challenge dose-response experiment, 18 additional guinea pigs for RWP challenge and fluticasone treatment, and 8 naïve guinea pigs (all groups underwent SFFV determination as the final procedure). Female guinea pigs were not used in these experiments, to avoid potential issues with estrus hormonal cycling and the inflammatory process being studied. Animals were anesthetized during all experiments. Inhaled isoflurane was used for the high-resolution imaging experiments (isoflurane mixed with air to a final concentration of 2% of isoflurane), and sodium pentobarbital (35–40 mg/kg) was used during all direct sinus measurement procedures, described below. At the end of all experiments performed, guinea pigs were humanely euthanized with an overdose of pentobarbital sodium (150 mg/kg; FATAL-PLUS; Med-Vet International). All animal care and experimental procedures were in accordance with the National Institutes of Health (NIH) guidelines and were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Allergic sensitization and challenge.
Research-grade RWP proteins (Giant and Short) were purchased from Greer. Guinea pigs (GPs) were sensitized with an intraperitoneal injection of RWP (100 μg of each protein) initially on day 0 and with a booster on day 7 (Fig. 1). On days 15–19, 16 GPs were given an intranasal challenge of RWP concentrations (0, 6, 12, and 22 μg/ml), delivered in sterile phosphate-buffered saline (PBS; Sigma). Typically, a total volume of 60 μl of RWP/saline solution was placed into each nostril during each RWP administration. For GPs being used as controls (0 μg/ml), 60 μl of PBS was given with the same timing as the RWP solution. Preliminary experiments (data not shown) indicated that full 100% sinus occlusion could be obtained with RWP concentrations ≥25 µg/ml. Further concentration-response experiments, described below, indicated that the optimal RWP concentration for the induction of a reversible allergic congestion response was 22 μg/ml, which was administered as described above.
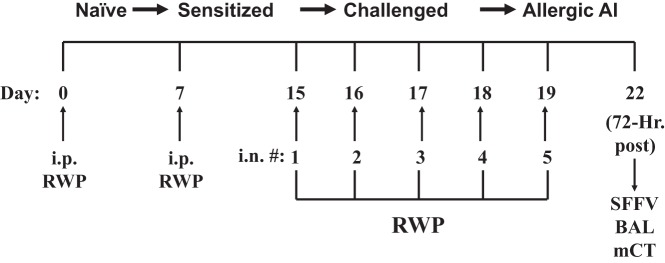
Fig. 1.
Allergy induction protocol used to induce allergic rhinosinusitis in guinea pigs with intranasal (i.n.) ragweed pollen (RWP) administration, progressing toward allergic airway inflammation (AI). Guinea pigs progressed toward allergic rhinosinusitis through initial systemic injections of RWP (intraperitoneal) followed by i.n. application of RWP over a 5-day period. In fluticasone-treated groups, the i.n. application of RWP occurred in the early morning, and the fluticasone treatment was applied i.n. in the late afternoon of each day. Bronchoalveolar lavage (BAL), sinus fluid fill-volume (SFFV), and microcomputed tomography (mCT) measurements were taken subsequently 72 h after the last i.n. RWP administration. #, Number.
Anti-inflammatory drug treatment.
To provide both a test of the ability of the SFFV method to measure changes in RWP-associated sinus congestion and to determine whether steroid dose-dependent effects on congestion could be observed, fluticasone (as Flonase; GlaxoSmithKline), a commonly used anti-inflammatory intranasal steroid drug, was given once per day intranasally, 6–8 h after each intranasal challenge with RWP, as described above. RWP was given at a highest concentration of 22 μg/ml, and fluticasone was given at concentrations 0, 25, 50, 75, and 100 μg to groups of four or five GPs. This approach allowed a testing protocol in which treatment for congestion was tested in a time domain occurring after the initial exposure to allergen in a therapeutic fashion, rather than a prophylactic one, in which treatments are given before all exposures to allergen. This approach also allowed for establishment of the directly measured allergic congestion and resolution responses for comparison with the noninvasive sinus congestion assessment methods.
Sinus fluid fill-volume measurements.
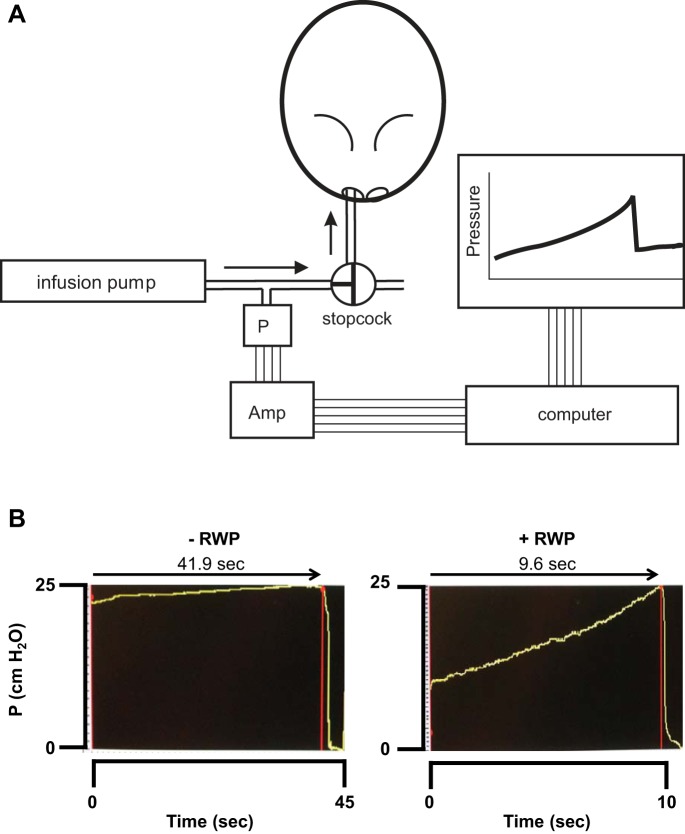
On day 22, 72 h after the final intranasal instillations described above, sinus congestion was measured directly using an SFFV assessment method, modified after that described by Straszek and Pedersen (44). Decapitation under surgical levels of sodium pentobarbital anesthesia was performed using a guillotine, and the blood was allowed to drain from the head and nasal cavity region. The SFFV was measured as a fluid-volume displacement that was dependent on the measured total sinus air-space volume. Briefly, the head of the guinea pig was mounted nostrils downward, with tubing inserted into the nostril and sealed in place with cyanoacrylate glue, to form a leak-proof seal. A digital constant-infusion syringe pump (Harvard Apparatus) was connected to the nostril tube and was used to deliver a nondiffusible liquid, perfluorocarbon (Alcon), into the sinus air spaces at a constant rate (Fig. 2A). A nondiffusible noncompressible liquid was necessary so that 1) the fluid would not diffuse across the sinus epithelium and all of the fluid volume delivered could reasonably be assumed to remain within the sinus cavities and 2) the fluid would not alter the pressure measurements due to compression as the pump pressure was applied to deliver the fluid into the sinus air spaces. A calibrated pressure transducer connected to an IBM PC-compatible computer was mounted on a side port of the delivery tubing from the pump to provide a measurement of the applied pressure within the setup, as pump infusion proceeded. The analog direct current pressure signals were acquired with an analog-to-digital card (National Instruments) and converted to digital signals that were visualized, recorded, and analyzed using digital signal analysis software (BioBench; National Instruments). The pressure signal was monitored visually over time, with a sudden pressure drop being identified as the point at which the infused volume reached the rearmost portion of the nasal airways, at the nasopharynx, and the sinus air spaces were completely full (Fig. 2B). With the known constant pump infusion rate and the elapsed time from the initiation of delivery to the pressure breakpoint, the resultant timed volume was quantified as the SFFV. All SFFV values obtained were normalized by the body weight of each guinea pig to control for variations in sinus sizes and air-space volumes using animal weight as an acceptable surrogate of body size and, thereby, head and sinus size.
Fig. 2.
A: schematic of sinus fluid fill-volume measurement setup, incorporating constant-flow infusion pump delivering perfluorocarbon liquid directly into nasal sinuses of guinea pig, with pressure transducer in delivery line to monitor pressure development and release, with achievement of sinus filling. Amp, direct current (DC) amplifier feeding DC pressure signal to personal computer from pressure transducer (P) via analog-to-digital conversion board, with digital readout from data display and acquisition program, shown on computer monitor. B: screenshots of typical pressure tracings recorded during the sinus fluid fill-volume experiments. Left: tracing of pressure development and release in guinea pig with no administration of ragweed pollen (RWP); elapsed time of pressure development was ~42 s, indicating a long duration of sinus filling, consistent with a high volume in uncongested sinuses. Right: tracing of pressure development and release in guinea pig treated with intranasal (i.n.) administration of RWP; elapsed time of pressure development was <10 s, indicating a brief duration of sinus filling, consistent with a low volume in congested sinuses. Note the difference in time scale at the bottom of each tracing.
Microcomputed tomography.
Microcomputed tomography (micro-CT) was used to acquire 2-D and 3-D images to capture stages of allergic congestion and resolution with drug treatment using a Siemens Inveon CT/PET/SPECT small-animal scanner. Before this procedure, guinea pigs were anesthetized with isoflurane diluted with air to 2%. The sinuses of the guinea pigs were imaged at 72 h after final intranasal RWP challenge and fluticasone treatment (day 22). The micro-CT imaging parameters were set with an exposure time of 1,000 ms (1 s) per projection, 70-kV X-ray source voltage, 500-µA X-ray source current, 107-μm isotropic resolution, 360° rotation with 520 projections, 54.85 × 82.27-mm field of view at scanning, and 512 × 512 × 768 voxels of reconstructed image. The software packages used were the Siemens Inveon Acquisition Workplace (for performing the scan) and Inveon Research Workplace (for image postprocessing). Images collected in this fashion resulted in files that averaged ~400 megabytes, typically averaging a total of 768 axial slices, at a resolution of 100 μm per slice, with no gaps between slices. The radiodensity scale was calibrated with air as −1,000 Hounsfield units (HU), and water as 0 HU. The whole sinus air volumes were calculated using the free air space of image densities in the range of −1,000 to −200 HU, beyond which, the range of −200 to −50 HU was considered to be high-density tissue, such as fat, with bone and cartilage considered to be beyond 0 HU. Therefore, radiodensity signals greater than −200-HU range were not included in the image and subsequent calculations of air space.
The 2-D linear distance-based sinus congestion assessment method relied on identification of common anatomic bony landmarks across animals in which the nasal septum and rear upper molars were used as common landmarks. Three main sinus regions were identified: 1) the proximal sinus cavity (nearest the nares), defined as the cavity space from the tip of the nares to the first incisor tooth; 2) the medial sinus cavity, identified as the cavity space caudal to the proximal sinus cavity, marked from the first incisor tooth to the most rearward molar tooth; and 3) the distal sinus cavity, identified as the cavity space caudal to the medial sinus space, marked from the most rearward molar tooth to the first appearance of the eye socket. The sinus air-space width measurement was taken as the cumulative length of air spaces across the total length of the linear distance within the proximal sinus cavity region, at a reference image density of −400 HU, to ensure that bony and cartilaginous structures were accounted for, in the determination and measurement of air-space widths.
We also compared predetermined singular cross-sectional sinus image slices, taken from the 3-D full sinus image, at specified sinus longitudinal length intervals across animals, within each of the three sinus regions outlined above, to determine whether cross-sectional slice depths across the longitudinal full sinus axis could be reproducibly assessed and compared for similar evidence of sinus inflammation and resolution. With the use of the static anatomic landmarks of the tip of the nares and the eye socket, the full longitudinal length of the sinus was normalized to represent 100%, with intermediary distances represented as 25, 50, and 75% of full longitudinal length. This approach allowed us to account for differences between guinea pigs in head lengths, and, therefore, sinus lengths, to arrive at the same relative depth of the longitudinal sinus axis, across animals.
Lower airway allergic inflammation assessment.
Because of 1) the invasive nature of the SFFV method, 2) the necessity to maintain an undisturbed sinus for the measurement of allergic sinus congestion and drug-associated resolution, and 3) the fact that sinuses treated with perfluorocarbon were unsuitable for subsequent sinus wash analyses, we utilized bronchoalveolar lavage (BAL) in several groups of guinea pigs to assess the degree of airway leukocyte infiltration as a further index of allergic airway inflammation associated with intranasal RWP administration. For comparison of this index of inflammation with other animal models using intranasal administration of allergic agents, we also administered intranasal ovalbumin in several groups of animals, on the same schedule as was used for RWP, outlined above. At the end of those experiments, guinea pigs were euthanized by pentobarbital sodium injection of 150 mg/kg, and the tracheas were cannulated with polyethylene tubing (#4) connected to a 10-ml syringe, into which two administrations of 1 ml of ice-cold sterile phosphate-buffered saline (PBS) per 100 g body wt were instilled into the lungs, and recovered by gentle aspiration. The collected BAL fluid was centrifuged for 10 min at 4°C at 120 g (at 1,000 rpm) to isolate cells, which were then resuspended, pooled, and spun onto slides using a Cytospin 4 cytocentrifuge (Thermo Fisher). The slides were stained with hematoxylin-eosin stain (CAMCO Stain Pak; Fisher Scientific/Cambridge Diagnostic Products), and eosinophils were counted under standard light microscopy at ×40 magnification.
Statistical analyses.
SigmaPlot graphical/statistical software (v. 12 and 13; Systat) was used to calculate descriptive statistics and perform statistical analyses. Independent sample t-tests were performed on BAL leukocyte data, to determine differences between treatment groups, with P values <0.05 considered to be significant. Normality tests were run on all data tested; if data did not pass the normality tests, then the nonparametric equivalent test was performed, at the same level of significance. In the case of t-tests, the Mann-Whitney U test was performed. The SFFV and image data were analyzed using one-way ANOVA. With acquisition of a significant F-statistic by ANOVA, post hoc testing using the Student-Newman-Keuls method was applied to determine discrete differences between treatment groups. If the sinus data were found to fail the normality test, then the nonparametric Mann-Whitney rank sum test was applied, with significance taken at the level of P < 0.05.
RESULTS
Induction of allergic sinus congestion.
We observed a RWP concentration-dependent increment in sinus congestion (Fig. 3), evident as a significant decrement in SFFV values, which reached a maximum of a ~75% average decrement in SFFV, in comparing the control (0 µg/ml RWP) and the highest administered RWP concentration (22 µg/ml). This pattern of concentration-dependent induction indicated that the allergic congestion response was able to be reproducibly modulated to a desired level that could then be studied with imaging and could be reversed with administration of an anti-inflammatory drug, as described below.
Fig. 3.
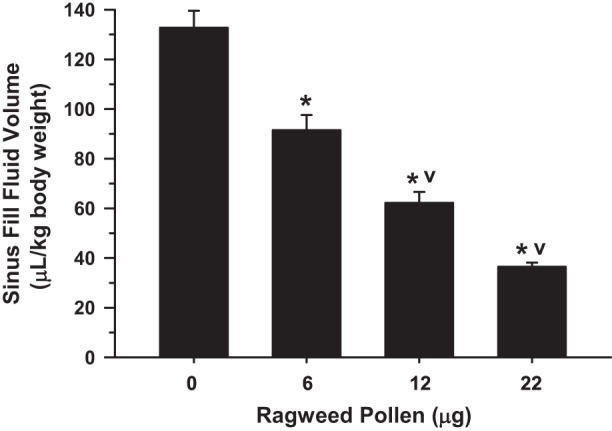
Sinus fluid fill-volume (SFFV) of guinea pigs with increasing intranasal ragweed pollen (RWP) administration. SFFV decreased maximally to an average of ~25%, indicating an increase in sinus congestion of nearly 75%. Values are means ± SE. *P < 0.05 compared with control (0, no RWP); vP < 0.05 compared with next lowest RWP treatment group; n = 4 guinea pigs/bar.
Airway inflammation: BAL cell analysis.
Leukocyte recruitment to the airways typically increases as part of the allergic airway inflammation response (11, 12, 24–26, 40), which provided a measure of the relative level of inflammation in our model of allergic rhinosinusitis. The BAL cell counts and patterns with RWP-induced allergic airway inflammation were comparable with those obtained with administration of ovalbumin (Fig. 4A), confirming the similarity of allergic inflammation within the airways with application of two different allergens.
Fig. 4.
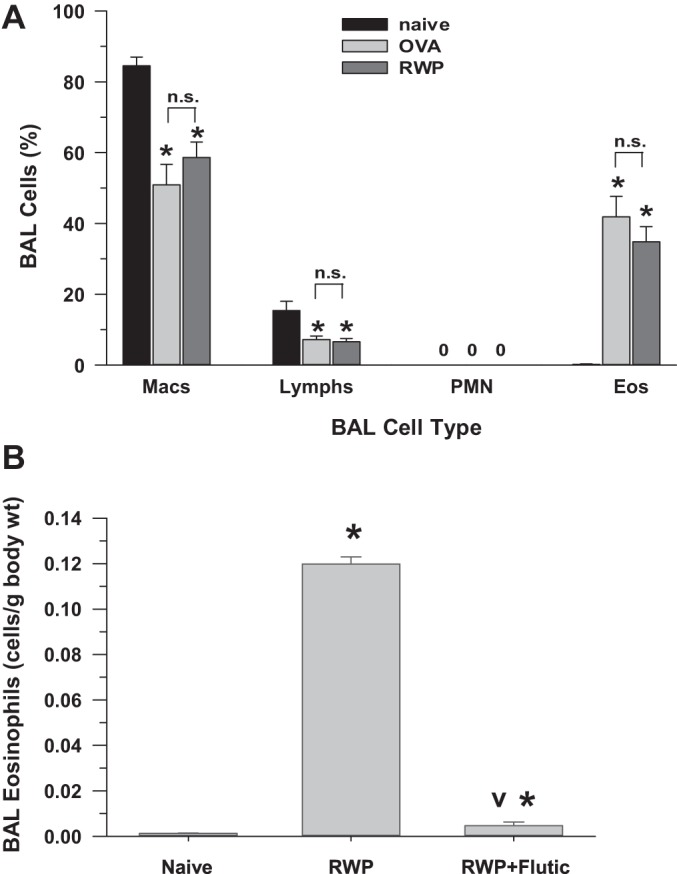
A: bronchoalveolar lavage (BAL) leukocyte cell differentials in guinea pigs, either naïve (no sensitization or challenge; n = 8) or with intranasal ovalbumin (OVA; 100 mg/ml; n = 7) or intranasal ragweed pollen (RWP; 22 µg; n = 10). Eos, eosinophils; Lymphs, lymphocytes; Macs, macrophages; PMN, polymorphonuclear leukocytes. Values are means ± SE. *P < 0.05 vs. naïve. 0, No cells observed; n.s., not significant. B: total eosinophil counts in naïve (n = 5), RWP-sensitized and -challenged (n = 5), and RWP + fluticasone (Flutic)-treated (100 µg; n = 5) guinea pigs; data are from naïve and RWP-exposed guinea pigs randomly selected from combined experiments to compare with the RWP + fluticasone experiments. Values are means ± SE. *P < 0.05 vs. naïve; vP < 0.05, RWP + fluticasone vs. RWP group.
With the induction of an allergic response in the RWP-challenged guinea pigs, the BAL eosinophil count was significantly increased compared with naïve guinea pigs, as shown in Fig. 4B. As expected, eosinophil counts in fluticasone-treated RWP-challenged guinea pigs were significantly decreased compared RWP-challenged guinea pigs, declining to counts nearly similar to naïve guinea pigs and demonstrating the resolution of allergen-associated airway inflammation with application of an intranasal anti-inflammatory steroid.
Anti-inflammatory drug effects on sinus congestion.
Fluticasone significantly decreased RWP-associated allergic sinus congestion in a concentration-dependent fashion, evident as stepwise increments in SFFV (Fig. 5). Specifically, fluticasone concentrations of 50–100 µg/ml were effective in progressively increasing SFFV, reaching a point of near-total reversal of the RWP-associated allergic sinus congestion with administration of 100 µg/ml, toward high SFFV values, which was not significantly different from that of naïve guinea pigs.
Fig. 5.
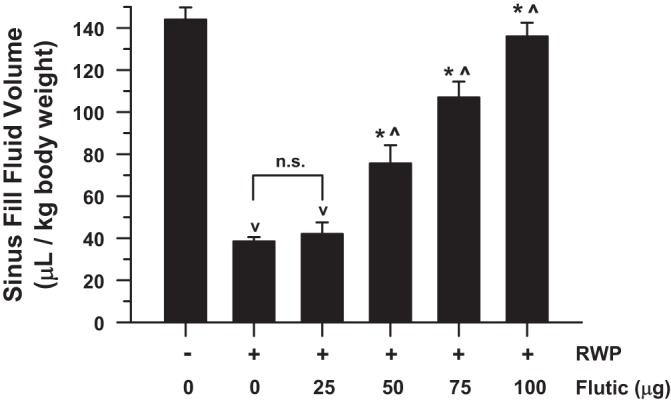
Sinus fluid fill-volume (SFFV) as a function of administered concentration of fluticasone in guinea pigs with ragweed pollen (RWP)-associated allergic sinus congestion. For comparison, far left bar indicates average SFFV values in naïve guinea pigs (no RWP or drug), which are of large magnitude, as would be expected with uncongested, untreated sinuses. Similar to Fig. 3, administration of RWP significantly decreased SFFV by ~75%, indicating significant allergic sinus congestion, compared with naïve guinea pigs. Fluticasone (Flutic) treatment significantly increased measured SFFV values in a concentration-dependent fashion, toward values similar to those observed in naïve animals. Values are means ± SE. vP < 0.05 compared with naïve group; *P < 0.05 compared with RWP alone; ^P < 0.05 compared with next lowest concentration of fluticasone. n.s., Not significant. Respective treatment group n’s for naïve (same as “0” in Fig. 3), RWP (same as 22 µg in Fig. 3), RWP + 25 µg of fluticasone, RWP + 50 µg of fluticasone, RWP + 75 µg of fluticasone, and RWP + 100 µg of fluticasone were 4, 4, 4, 5, 4, and 5.
2-D image width analyses of sinus congestion and treatment.
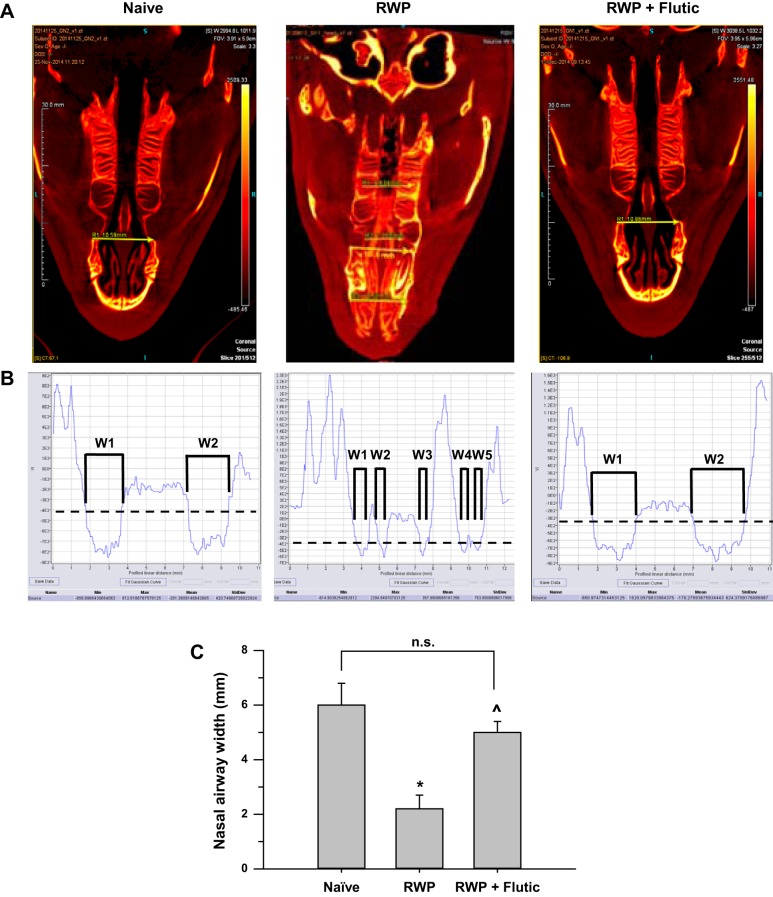
With the use of the linear analysis method outlined above, cumulative linear sinus widths were measured in naïve, RWP, and RWP + fluticasone groups, with representative images in Fig. 6 showing the drawn linear line segment (Fig. 6A), the image terrain/density analyses, as the density of tissue was followed from left to right along the line segment path in each 2-D image above (Fig. 6B), and the average linear sinus width measurements (Fig. 6C). The effect of slight differences in head alignment angles across images was minimized by drawing between bilateral landmarks with line segments that were perpendicular to the central axis of the head. With induction of allergic rhinosinusitis by RWP, linear sinus width was decreased to approximately one-third of that measured in naïve guinea pigs. With administration of fluticasone, linear sinus width was increased over that observed in the RWP group to an average width that was not statistically different from the naïve group. The pattern of results with the linear sinus width method (Fig. 6C) was similar in pattern to those obtained using the SFFV technique, as shown in Fig. 7C, suggesting that the linear sinus width method of 2-D image analysis tracked the pattern of changes in congestion with allergic induction and subsequent anti-inflammatory treatment.
Fig. 6.
Two-dimensional image analyses of sinuses of naïve, ragweed pollen (RWP)-treated, and RWP + fluticasone (Flutic)-treated guinea pigs quantifying cumulative linear width of nasal airway passages at a fixed bony landmark using a high-contrast coloration scheme to allow definitive identification of skull bone structures, cartilage, and air spaces (black), measuring across the linear yellow arrow for image densities of −200 Hounsfield units (HU) for soft tissue (cartilage), −800 HU for air-space openings, and +1,100 to +1,500 HU for bone. A: representative images indicating linear line segment placement and relative widths observed; note presence of significant mucous accumulation in the proximal and medial sinus spaces of RWP example, shown as strong yellow signal along midline of sinus passage, compared with heterogeneous yellow and black signals in the distal sinus area in the same image. Length scale within images shown at left is in millimeters; image voxel intensity (in HU) color band is shown at right, indicating air at bottom with dark color and bone at top with bright yellow color; virtual image slice number is indicated at lower right. B: image terrain/density analyses for each of the images shown above in A, with lower densities along the ordinate indicating air spaces and higher densities along the ordinate indicating dense bony and cartilaginous structures of the sinus. VI (ordinate), voxel intensity (in HU). Min, Max, Mean, and StdDev for VI signal running from 0 to 10–12 mm of profiled linear distance (abscissa) are shown at bottom of each picture in B. Dashed, black line indicates −400-HU image density reference point, at which cumulative width (W) measures were made, as shown for naïve (W1 + W2), RWP (W1 + W2 + W3 + W4 + W5), and RWP + fluticasone (W1 + W2). C: average cumulative linear widths (means ± SE) across groups. *P < 0.05 vs. naïve, ^P < 0.05 vs. RWP. naïve, n = 5; RWP, n = 4; RWP + fluticasone, n = 5. n.s., Not significant.
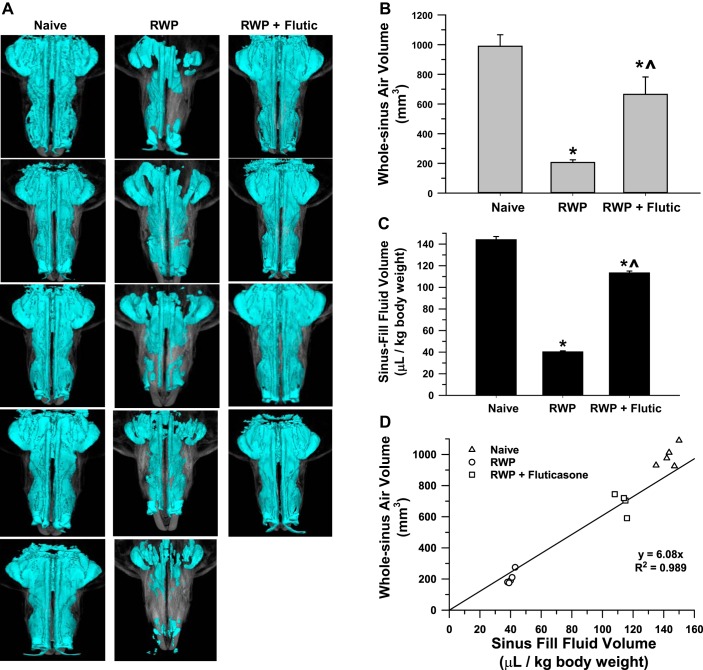
Fig. 7.
A: pictures of 3-dimensional whole sinus images of guinea pigs from naïve (n = 5), ragweed pollen (RWP)-challenged (n = 5), and RWP + fluticasone (Flutic; 100 µg; n = 4) groups. B: whole sinus air volume as measured by microcomputed tomography (micro-CT) in the same groups as indicated in A. Statistical significance is indicated as *P < 0.05 compared with naïve and ^P < 0.05, RWP + Flutic compared with RWP alone. C: sinus fluid-fill volume (SFFV) measurements in the same treatment groups as pictured in A; significance symbols as in B. D: association of sinus volume measured with the SFFV method and the whole sinus air volume measured by micro-CT for guinea pigs in A–C with linear regression equation (y = 6.08x) and correlation coefficient (r2 = 0.989) as shown; regression was constrained to pass through the origin of the plot; standard error and lower and upper confidence limits of slope were 0.38, 4.89, and 7.27, respectively.
3-D image whole sinus volume analyses of sinus congestion and treatment.
With the use of the 3-D image whole sinus volume analysis method outlined above, the sinus volumes were measured in naïve, RWP, and RWP + fluticasone groups with representative image slices of the 3-D images showing the air-space volume in blue color in each image (Fig. 7A). As shown in Fig. 7B, with induction of allergic rhinosinusitis by RWP, 3-D-imaged whole sinus air volume was decreased to approximately one-third to one-quarter of that measured in naïve guinea pigs. With administration of fluticasone, whole sinus volume was increased over that observed in the RWP group, to an average volume that approached that of the naïve group. The results with this method were similar in pattern to those obtained using the SFFV (Fig. 7C) and the 2-D linear width techniques (Fig. 6C), suggesting that the 3-D whole sinus airway image volume analysis method was accurate in tracking changes in congestion with allergic induction and subsequent anti-inflammatory treatment compared with the direct SFFV assessment method. Figure 7D shows the linear association of the SFFV and whole sinus air volume measurements as being along a line of positive slope in which low SFFV values were correlated with low whole sinus air volume values with RWP administration, as measured by micro-CT.
3-D image slice analyses of sinus congestion and treatment.
As a result of calculations and normalization of the full length of the guinea pig nasal sinuses, based on the 25, 50, and 75% length reference points described above, we were consistently able to locate region-based anatomic structures inside the guinea pig sinuses with reproducible precision. Accordingly, for each guinea pig, we back-calculated from the last slice that represented the 100% length to the slice numbers that represented the 25, 50, and 75% of full-sinus-length landmarks (moving rostral to caudal), as shown in the representative oblique relief images, in which the full-sinus image (Fig. 8A) is compared with the single cross-sectional image slices (Fig. 8B) from that same full-sinus image, at each specific sinus reference length. We found that this approach allowed us to relocate to the same sinus area within 0–3 slices in either the rostral or caudal direction, making our greatest experimental relocation error <1% (6-slice total variance/768 total slices) or a maximum sinus-length distance error of ±30 μm from the desired reference point. With practice, we found that we were typically either exactly on target or no more than 1 virtual slice (10 μm) from our desired target. Thus this approach allowed us to return to accurately, and thus monitor and assess, 1) the same sinus regions within each guinea pig, 2) the same relative sinus anatomic area across guinea pigs, and 3) treatment effects in the same relative anatomic sinus regions across guinea pig treatment groups.
Fig. 8.
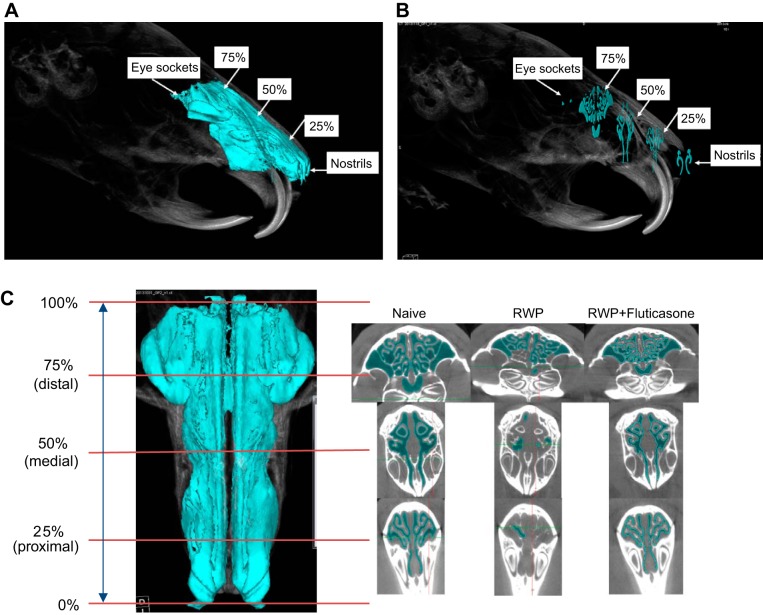
A: oblique-angle view of 3-dimensional (3-D) naïve guinea pig full-sinus image indicating anatomic and sinus-length landmarks used for microcomputed tomography (micro-CT) image slice analysis, with air-space volume indicated by blue coloration. Nostrils and eye sockets provided the proximal and distal whole sinus-length boundaries, from which the 25, 50, and 75% sinus-length landmarks were derived. B: same oblique-angle view as indicated in A, with micro-CT images of single slices obtained at 25, 50, and 75% sinus-length intervals and with nostril and eye socket slice images at sinus-length extremes for reference. C, left: 2-dimensional picture of the same naïve guinea pig full-sinus image with air-space volume as in Fig. 7, viewed axially (looking down directly on top of the head), to show the full length, and right-angle-to-longitudinal-axis register of the 25, 50, and 75% sinus-length slice landmarks, demarcating the proximal, medial, and distal sinus regions. Right: composite of micro-CT slices taken from 3-D full-sinus image at each length landmark for representative single guinea pigs from the naïve, ragweed pollen (RWP), and RWP + fluticasone treatment groups in which dark blue coloration was used to indicate air spaces within the respective slices at each level. Note 1) the similarity of sinus image topography across sinus landmark levels and guinea pigs in each group and 2) the relative lack of dark blue coloration within the image slices of the RWP examples, indicating reduced air-space volume and increased sinus congestion, compared with the naïve and RWP + fluticasone treatment examples.
The relative 25, 50, and 75% length landmark slice locations corresponding to the proximal, medial, and distal sinus regions, moving from bottom (nostrils) to top (eye socket area) of the image, are shown in Fig. 8C, illustrating a vertically oriented sinus image from a typical naïve guinea pig. For comparison, the resultant cross-sectional images at each length reference point are shown directly to the right, from three separate guinea pigs representative of each of the respective naïve, RWP, and RWP + fluticasone treatment groups. In these cross-sectional images, air space can be observed to be decreased at each sinus level with RWP, compared with naïve, and was increased with fluticasone treatment, toward air volume area fills similar to that of the naïve guinea pig. Although exemplary, further evidence of these differences is shown in a similar comparison of virtual 3-D slice images of three guinea pigs per treatment group with sections at each length level of the whole sinus (Fig. 9). These images are provided to show 1) the repeated similarity of images at each length level within the control and experimental groups, 2) the changes that can be observed repeatedly with RWP-induced allergic congestion at each length level, and 3) the repeated reversal of those changes at each length level with fluticasone treatment.
Fig. 9.
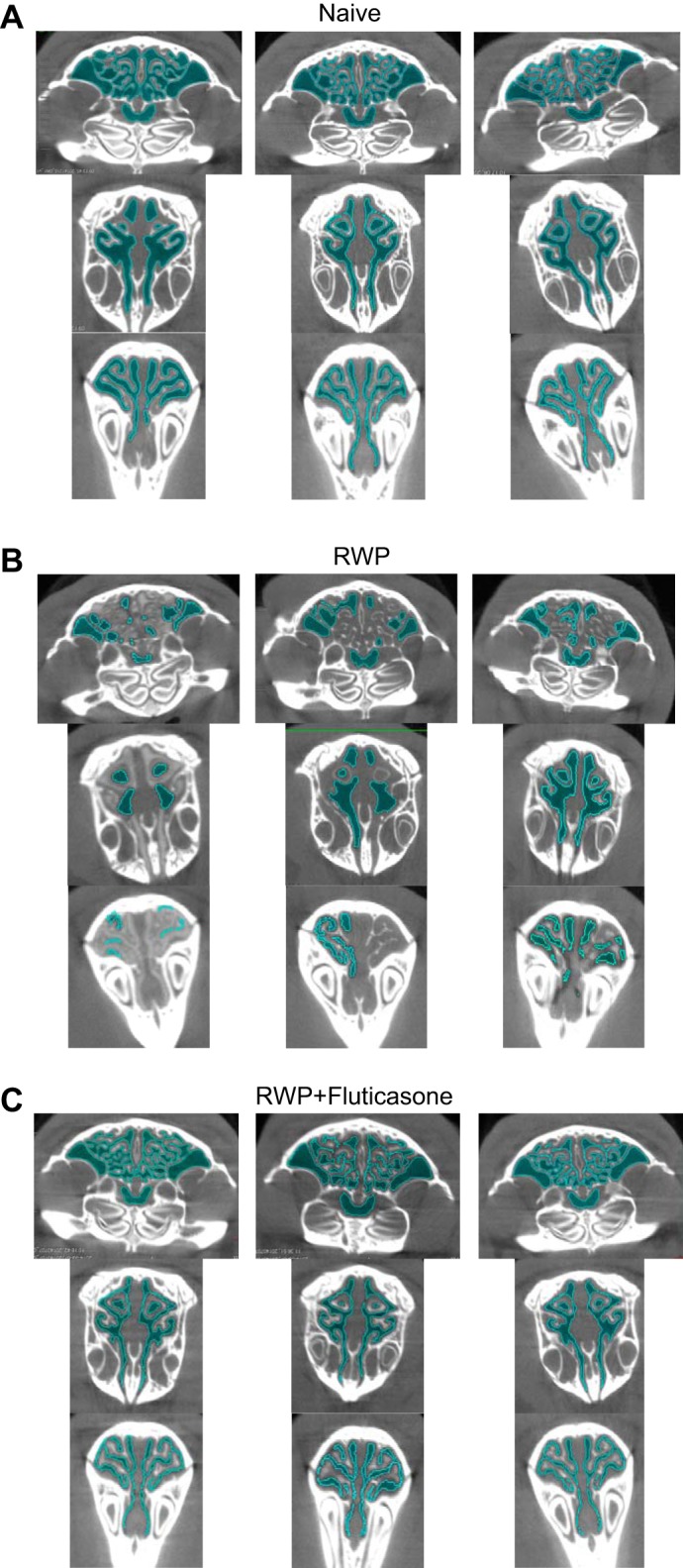
Three sets of microcomputed tomography (micro-CT) image slices taken from three whole sinus three-dimensional micro-CT images of guinea pigs from naïve group (A), ragweed pollen (RWP) group (B), and RWP + fluticasone group (C) to indicate assessment and comparison of sinus air-space area (darker blue coloration) and sinus perimeter (lighter blue coloration) demarcation. Images from the RWP-treated guinea pigs consistently demonstrated a decrease in both the darker and lighter blue coloration compared with those in the naïve and RWP + fluticasone treatment examples.
Finally, we measured both the sinus air-space cumulative area and the sinus surface perimeter within each 3-D slice at the 25, 50, and 75% sinus-length levels in guinea pigs from each of the naïve, RWP, and RWP + fluticasone groups. As shown in the images of Fig. 9, the calculated air-space area corresponded to the darker blue color of air space within each slice (as illustrated in Figs. 9 and 10), and the calculated sinus surface perimeter corresponded to a lighter blue highlight of the boundary of each air space. Visual inspection of the darker blue coloration in those images verifies the relatively open sinus space in the naïve group, much less darker blue coloration in the RWP group (corresponding to allergic sinus congestion), and increased darker blue coloration in the fluticasone-treated group. With the use of these methods of assessment, we plotted average sinus air-space area values (Fig. 10A), average sinus surface boundary perimeter values (Fig. 10B), and the ratio of area to perimeter for each treatment group (Fig. 10C) at each sinus slice level. As shown with the other methods of 3-D image assessment and the “gold-standard” SFFV method, the patterns of sinus area and perimeter changes are consistent with what would be expected with RWP-associated induction of allergic sinusitis and its amelioration with administration of an anti-inflammatory steroid. However, visual inspection and comparison with the results from the SFFV method and the whole sinus air-space volume assessments (as shown in Fig. 7) suggest that the greatest fidelity of the sinus air-space area, surface perimeter, and area-to-perimeter ratio in tracking the allergic sinus congestion and its reversal with anti-inflammatory treatment occurs within the proximal sinus at the 25% sinus-length level.
Fig. 10.
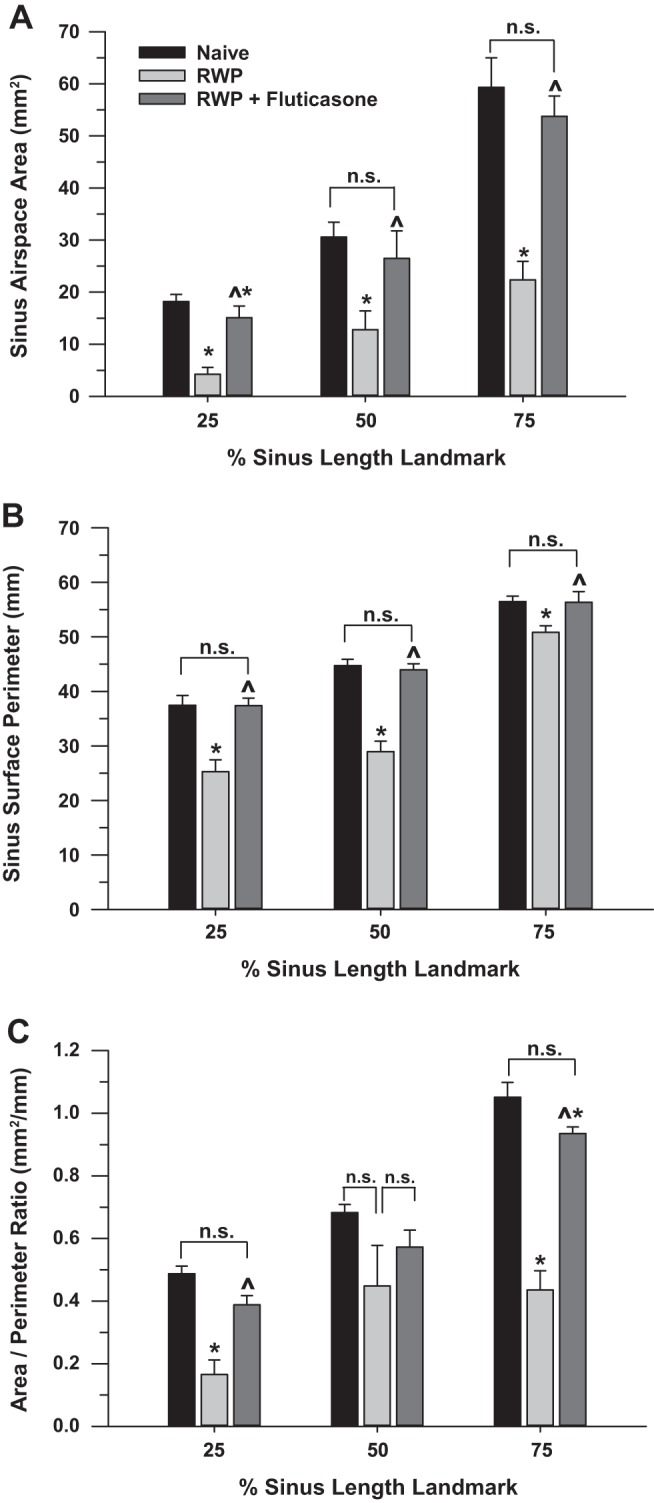
A: measurements of sinus air-space area from 25, 50, and 75% sinus-length landmark slices, as shown in Fig. 8 (landmark levels) and Fig. 9 (areas of darker blue), in naïve (n = 5), ragweed pollen (RWP; n = 5), and RWP + fluticasone (n = 4) treatment groups. Data are means ± SE. *P < 0.05 compared with naïve; ^P < 0.05, RWP + fluticasone compared with RWP. n.s., Not significant as compared by ANOVA. B: measurements of sinus surface perimeter from 25, 50, and 75% sinus-length landmark slices, as shown in Fig. 8C (levels shown at left; perimeter outline shown as areas of lighter blue at right; also shown in Fig. 9), in the same naïve, RWP, and RWP + fluticasone treatment groups as in A. Data are means ± SE; significance symbols as in A. C: ratios of sinus air-space area per sinus perimeter, derived from 25, 50, and 75% sinus-length landmark slices, as shown in Figs. 8C and 9 (areas of darker blue and lighter blue), in naïve, RWP, and RWP + fluticasone treatment groups, as in A. Data are means ± SE; significance symbols as in A and B.
DISCUSSION
The main findings of present studies were 1) 2-dimensional micro-CT methods utilizing nasal width measurements, 3-D micro-CT whole sinus volume measurements, and 3-D micro-CT sinus image slice analyses all provided assessments of allergic sinus congestion that were similar to those obtained with the direct gold-standard SFFV method, 2) a normalized 3-D micro-CT image slice location method allowed a reproducible degree of accuracy in analyses of inflammation and its resolution both across individuals and experimental groups, and 3) micro-CT 3-D image slice analyses provided assessments of both sinus air-space area and surface perimeter that were likewise consistent with the SFFV, 2-D linear width, and 3-D whole sinus air volume methods of allergic sinus congestion and resolution measurement.
Guinea pig model of allergic rhinosinusitis.
Although investigation of allergic sinus congestion has been undertaken in other animal models, such as mice and rats, the guinea pig was considered to be superior for the present studies due to its size and its similarity of allergic responses and symptoms to those of humans. Consistent with allergic airway inflammation in human asthma, we verified the presence of allergic airway inflammation in our guinea pig model by quantification of the BAL cell differential and observation of RWP-associated eosinophilia, which was resolved with administration of fluticasone (Fig. 4). Those findings in the lower airways were consistent with a prior report in which eosinophilia and its resolution with fluticasone was observed in the nasal lavage and mucosa of guinea pigs administered ovalbumin (2). Although it is possible that there could be a lack of association between sinus airway and lung airway inflammation in our model, we note that many animal models of lung airway inflammation are induced through intranasal application of allergens and that the inflammation of both the upper and lower airways often occur together in human asthma. Anecdotally, we often observed enhanced mucus secretion with runny noses and sneezing in our animals that had been given RWP intranasally, similar to that reported previously in guinea pigs (2, 27) and indicative of the late-phase histamine-associated allergic response (51) and symptoms observed in humans with allergic rhinosinusitis (52).
Sinus fluid-volume method as a gold standard.
There exists a number of methods from which to choose to measure sinus airway congestion; they range from noninvasive to minimally invasive and/or fully invasive. One minimally invasive and nonphysiological method of nasal patency assessment in humans (i.e., no surgical cutting or penetration of skin or other structural barriers) is acoustic sinus rhinometry (28). However, this method requires complex mathematics, subject cooperation with breath-hold maneuvers, ambient temperature control, and noise control and is often limited due to variations in structure of nasal cavities, besides being based on aspects of subjective assessments (6a, 10, 36), some of which require a “standard nose” with which to compare (17), which is clearly unsuitable for investigations in guinea pigs. Furthermore, due to the tortuosity of sinus structures, the measurement variation obtained with this sound-based method can be significant due to heterogeneity of reflected sound waves when irregular structures within the sinuses are encountered during the measurements. As shown by the 3-D image slices in Figs. 9 and 10, this would be particularly true for the distal sinus regions. Indeed, a series of studies comparing acoustic rhinometry with the fluid displacement method in dogs, cats, guinea pigs, and rats indicated that acoustic rhinometry underestimated sinus volume by an average of approximately 15–30% across those species (44, 45). Although noninvasive, for the reasons above, we judged the acoustic rhinometry method to be unacceptable as a gold standard for our purposes, against which to compare results from our noninvasive micro-CT methods.
Another noninvasive technique is magnetic resonance imaging (MRI) assessment, which has advantages similar to micro-CT of being repeatable and nonterminal. However, a study of MRI in comparison with the fluid displacement method in dogs and cats indicated that cross-sectional area of the sinus lumen was limited by the fact that its definition was dependent on subjective choices made by the image assessor, making MRI unsuitable as a gold standard of sinus measurement (45). Although we had obtained some interesting and congruent data with the 2-D linear cumulative-width measures of micro-CT images (Fig. 6), we found this same situation to be true regarding the line segment placement, which is one reason that we favored the whole sinus 3-D volume assessments.
Two additional more invasive methods for the assessment of sinus congestion are the use of nasal sinus casts (39, 43) and histological approaches (35), which are more conclusive than acoustic rhinometry but can be confounded by preparation issues such as deformation of compliant structures and histological sectioning artifacts and are likewise terminal in nature. Thus we selected the invasive but direct method of SFFV measurement as our gold-standard method for these studies.
Reliability of micro-CT sinus imaging methods.
As suggested above, a major advantage of the micro-CT imaging methods was their noninvasive, nonterminal nature. It was remarkable that each of the CT imaging techniques we utilized demonstrated a similar sinus congestion and resolution pattern that essentially equated nasal airway width (Fig. 6), whole sinus airway volume (Fig. 7), sinus air-space area (Fig. 10A), and sinus surface perimeter (Fig. 10B) with direct measurements of SFFV (Fig. 7). This gave us confidence that noninvasive image-based air volume measurements could be considered equivalent to direct fluid-volume measurements of sinus congestion.
Although we were able to show concordance of allergic sinus congestion and resolution results with the SFFV measurements using the 2-D linear method, the 3-D whole sinus volume method, and the 3-D image slice methods (Figs. 6 and 7), there were some limitations encountered as well. For example, the 2-D linear approach for measuring cumulative sinus width was applied only to the proximal sinus area, where relatively unambiguous bony anatomic landmarks were reliably evident and of similar sizes and shapes across guinea pigs in the age range we studied. The age of the guinea pig was an important surrogate of guinea pig size and growth, which can modify apparent width measurements, also adding a level of variance to such measurements. The 2-D linear approach also relied on the subjective skill and experience of the image operator to sample reliably the sinus length and location at a comparable depth across guinea pigs sampled, adding another potentially confounding variable to the reproducibility of this measurement. Thus, although the 2-D linear method obtained results in the proximal sinus that were consistent with the SFFV method, it had some characteristics that limited its consideration as an optimal image assessment method.
The slice assessment method utilizing normalized sinus-length location within the 3-D whole sinus images was remarkably accurate, from the perspective that the same relative sinus depth from the nostrils to the eye sockets could be reliably located both within a single guinea pig and across multiple guinea pigs (Figs. 8 and 10). This method likewise required some practice but was less ambiguous than the 2-D linear width method due to the reliable mathematical nature of the normalized length location approach and the consistent image slice thickness for each whole sinus image. An important aspect of this method was that the sinus surface perimeter, or sinus boundary, was quantifiable and demonstrated changes concordant with the other congestion assessment methods and particularly the SFFV data (Figs. 7, 9, and 10), which could be important in characterization of sinus airway inflammation and disease.
Of the three micro-CT image assessment methods, the 3-D whole sinus volume measurement approach offered the least ambiguous and most directly comparable results with the direct SFFV method. This is likely because both methods are essentially volume integrations of the whole sinus air space, which decreases with congestion and increases with resolution of congestion. Furthermore, as suggested by the illustration in Fig. 7, the 3-D whole sinus volume image also provides the most visually apparent changes in sinus congestion with the induction of allergic response and its treatment with an anti-inflammatory drug, which is a desirable characteristic of an image-based method of quantification. Thus, although some additional information is obtainable with the other image-based approaches, we judged the 3-D whole sinus volume method as providing the most reliable overall determinations of sinus congestion consistent with allergic rhinosinusitis.
Other methodological considerations.
Micro-CT offers a noninvasive, cost-effective approach allowing imaging of hard structures (e.g., bone and other tissue making up the sinuses), providing high sensitivity and high spatial resolution. This X-ray-based imaging tool can provide good resolution in a time-efficient manner while facilitating high throughput of analyses (7, 9, 15, 20, 38). However, two potential limitations of micro-CT are radiation dose and the somewhat low resolution of a number of soft-tissue structures (18, 19, 48). With refinement of the technique, it might be possible to focus attention on some of the resolvable sinus soft-tissue changes with allergic rhinosinusitis and its treatment. However, in the present study, soft-tissue modifications were not focused on, and the resolution of the scan of the nasal cavity structures was sufficient to distinguish among bone, cartilage, and air space.
While a necessary concern, the radiation dose would not be considered cumulatively damaging in the way that we typically applied micro-CT during one preterminal sinus imaging session. Micro-CT applied in small animals is generally thought to be harmful only as it relates to sequential and repeated exposures over compressed time intervals, such as days to weeks. Specifically, radiation from repeated micro-CT exposures could ultimately influence animal survival or tumor growth in small animals, such as mice (53), in which the relative radiation dose per total body mass can be high, compared with much larger guinea pigs. It has been reported that a lethal dose (LD) for 50% of exposed mice within 30 days (LD50/30) can range between 5.0 and 7.6 Gy and that a typical whole body radiation dose for a 3-D micro-CT can range from 0.017 to 0.78 Gy (7). At the high end of that typical range (0.78 Gy), an estimated cumulative LD50/30 of 7.6 Gy would theoretically be reached after ~10 closely timed successive whole body micro-CT imaging sessions in mice. Furthermore, rodents can repair alterations from sublethal doses of radiation in several hours (32) and have been reported to demonstrate no adverse effects within the lower airways during longitudinal micro-CT studies (8).
Presumably, guinea pigs would be able to tolerate >10 sequential micro-CT exposures before reaching LD50/30 radiation levels, owing to their larger size. In support of this probability, it has also been reported that the skin of mice and rats can absorb as much 0.045 and 0.028 Gy, respectively, during 1 micro-CT whole body scanning session but that the internal organs receive significantly lower doses, typically averaging <0.035 and <0.024 Gy in mice and rats, respectively (29). Thus, based on body mass and size scaling, with a typical young adult mouse weighing 20–30 g, a typical young adult rat weighing 300–500 g, and our studied young adult guinea pigs weighing 600–900 g, it would be expected that the skin of guinea pigs would cumulatively absorb <0.028 Gy in 1 micro-CT exposure and their internal organs even less than that amount during 1 micro-CT imaging session.
CT is commonly used in human clinical practice at dosages ranging from 0.005 to 0.070 Gy for interventional procedures, 0.003–0.020 Gy for most nuclear procedures, and 0.002 Gy for head CT analyses, compared with the average annual background radiation exposure of ~0.3 Gy (24a). The acute radiation dosage with a single clinical scan of sinuses is comparatively low, reported to range as low as 0.0005–0.006 Gy (2a) and 0.0029–0.0316 Gy (19a); cumulative doses, particularly for a surveillance scan that may be done at most once a year, would be even less. For patients with chronic disorders, such as severe rhinosinusitis, a major issue is induction of cataracts with radiation of the lens of the eye during serial CT imaging of paranasal sinuses, typically considered to occur at levels of 0.250 and 5.0 Gy for pediatric and adult patients, respectively (2a) but which can be avoided with proper shielding (19a). Particularly for adults, sinus CT scans are, therefore, considered to be of minimal risk for the induction of tissue pathology and cancer, especially compared with the relative benefit of accurate and personalized sinus diagnoses and therapy.
Comparisons with other studies of sinus congestion.
The anatomy and physiological responses of the guinea pig nasal cavities have been of interest to biomedical scientists as far back as 1948, with the proclamation that the guinea pig represented the ideal experimental animal for research work within the nasal cavity (21, 37). Our results obtained with 3-D whole sinus image analyses (Figs. 7 and 8) as well as our 3-D image slices (Figs. 8–10) compare favorably with a prior study using 2-D and 3-D CT imaging techniques in guinea pigs (33). Our 3-D image slices also compare favorably with older histological sections of the guinea pig sinuses (21), suggesting the practicality of the use of micro-CT as a noninvasive imaging method to assess sinus topography and alterations.
Our findings of significant and, in some cases, near-complete reversal of allergic sinus congestion with intranasal fluticasone administration, as assessed by all of the methods we employed, were strongly consistent with a prior study in guinea pigs in which fluticasone was reported to result in complete inhibition of congestion (2). Those data, considered together with the lower airway indices of inflammation and its resolution with fluticasone, further enhanced our confidence in the micro-CT approach as being valid for testing of drug treatments employed in the treatment of allergic rhinosinusitis.
Summary.
Our results indicate that the noninvasive, nonterminal micro-CT sinus air-space volume measurement method can provide allergy-associated sinus congestion data consistent with the more invasive and terminal directly measured SFFV method. The two methods produced data that were strongly associated when measuring both the degree of allergy-associated congestion induction and its resolution with administration of a classic anti-inflammatory steroid. Furthermore, we found that normalization for relative animal size differences and the use of consistent anatomic landmarks could be utilized to ensure reproducibility of micro-CT measurements across individuals and the subsequent identification of treatment effects. Taken in aggregate, the present study provides evidence that the micro-CT method can be used to measure accurately IgE-associated sinus allergic responses, suggesting a potential for utilization of micro-CT as an investigative tool in drug testing and mechanistic studies of allergic rhinosinusitis.
GRANTS
This research was supported by National Institute of Environmental Health Sciences Grants T32-ES-007254 and P30-ES-006676, the Sealy Center for Molecular Medicine, the Sealy Center for Environmental Health and Medicine, the University of Texas Medical Branch Institute for Translational Sciences and Division of Pulmonary, Critical Care & Sleep Medicine, and the Brown Foundation.
DISCLOSURES
W. C. Spear and B. T. Ameredes received grant funding from Alcon Research, Ltd. and the Forest Research Institute in support of a portion of this work.
AUTHOR CONTRIBUTIONS
B.T.A. conceived and designed research; J.L., W.C.S., and I.P. performed experiments; J.L., W.C.S., and B.T.A. analyzed data; J.L., W.C.S., I.P., M.M., and B.T.A. interpreted results of experiments; J.L., W.C.S., and B.T.A. prepared figures; J.L., W.C.S., and B.T.A. drafted manuscript; J.L., W.C.S., I.P., M.M., and B.T.A. edited and revised manuscript; J.L., W.C.S., I.P., M.M., and B.T.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Spotswood D. Miller for assistance with experiments and Drs. Sanjiv Sur, William J. Calhoun, and John Yanni for experiment consultation and advice.
REFERENCES
- 1.Bachert C, Marquardt U, Korte M. IgE-positive mast cells play a central role in nasal allergic disease. Am J Rhinol 4: 215–219, 1990. doi: 10.2500/105065890782009299. [DOI] [Google Scholar]
- 2.Bahekar PC, Shah JH, Ayer UB, Mandhane SN, Thennati R. Validation of guinea pig model of allergic rhinitis by oral and topical drugs. Int Immunopharmacol 8: 1540–1551, 2008. doi: 10.1016/j.intimp.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 2a.Bang M, Choi SH, Park J, Kang BS, Kwon WJ, Lee TH, Nam JG. Radiation dose reduction in paranasal sinus CT: with feasibility of iterative reconstruction technique. Otolaryngol Head Neck Surg 155: 982–987, 2016. doi: 10.1177/0194599816664335. [DOI] [PubMed] [Google Scholar]
- 3.Belvisi MG, Birrell MA, Khalid S, Wortley MA, Dockry R, Coote J, Holt K, Dubuis E, Kelsall A, Maher SA, Bonvini S, Woodcock A, Smith JA. Neurophenotypes in airway diseases. Insights from translational cough studies. Am J Respir Crit Care Med 193: 1364–1372, 2016. doi: 10.1164/rccm.201508-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet J, Bachert C, Bernstein J, Canonica GW, Carr W, Dahl R, Demoly P, Devillier P, Hellings P, Fokkens W, Klimek L, Lieberman P, Meltzer E, Price D, Ryan D, Wahn U. Advances in pharmacotherapy for the treatment of allergic rhinitis; MP29-02 (a novel formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) fills the gaps. Expert Opin Pharmacother 16: 913–928, 2015. doi: 10.1517/14656566.2015.1020789. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O’Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D; World Health Organization, GA2LEN; AllerGen . Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy 63, Suppl 86: 8–160, 2008. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 6.Buels KS, Jacoby DB, Fryer AD. Non-bronchodilating mechanisms of tiotropium prevent airway hyperreactivity in a guinea-pig model of allergic asthma. Br J Pharmacol 165: 1501–1514, 2012. doi: 10.1111/j.1476-5381.2011.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Chang CC, Incaudo GA, Gershwin ME (). Diseases of the Sinuses: A Comprehensive Textbook of Diagnosis and Treatment (2nd ed.). New York; Heidelberg, Germany; Dordrecht, Netherlands; London: Springer, 2014. doi: 10.1007/978-1-4939-0265-1. [DOI] [Google Scholar]
- 7.Clark DP, Badea CT. Micro-CT of rodents: state-of-the-art and future perspectives. Phys Med 30: 619–634, 2014. doi: 10.1016/j.ejmp.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detombe SA, Dunmore-Buyze J, Petrov IE, Drangova M. X-ray dose delivered during a longitudinal micro-CT study has no adverse effect on cardiac and pulmonary tissue in C57BL/6 mice. Acta Radiol 54: 435–441, 2013. doi: 10.1177/0284185113475608. [DOI] [PubMed] [Google Scholar]
- 9.Dudak J, Zemlicka J, Karch J, Patzelt M, Mrzilkova J, Zach P, Hermanova Z, Kvacek J, Krejci F. High-contrast X-ray micro-radiography and micro-CT of ex-vivo soft tissue murine organs utilizing ethanol fixation and large area photon-counting detector. Sci Rep 6: 30385, 2016. doi: 10.1038/srep30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdogan M, Cingi C, Seren E, Cakli H, Kezban Gürbüz M, Kaya E, Incesulu A, Ozudogru E, Kecik C. Evaluation of nasal airway alterations associated with septorhinoplasty by both objective and subjective methods. Eur Arch Otorhinolaryngol 270: 99–106, 2013. doi: 10.1007/s00405-012-1974-y. [DOI] [PubMed] [Google Scholar]
- 11.Florsheim E, Yu S, Bragatto I, Faustino L, Gomes E, Ramos RN, Barbuto JA, Medzhitov R, Russo M. Integrated innate mechanisms involved in airway allergic inflammation to the serine protease subtilisin. J Immunol 194: 4621–4630, 2015. doi: 10.4049/jimmunol.1402493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol 34: 398–409, 2013. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet 378: 2112–2122, 2011. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 15.Hafeez S, Huddart R. Advantages and limitations. In: PET/CT in Radiotherapy Planning, edited by Chua S. Cham, Switzerland: Springer, 2017, p. 33–38. doi: 10.1007/978-3-319-54744-2_6. [DOI] [Google Scholar]
- 16.Hellings PW, Fokkens WJ, Akdis C, Bachert C, Cingi C, Dietz de Loos D, Gevaert P, Hox V, Kalogjera L, Lund V, Mullol J, Papadopoulos NG, Passalacqua G, Rondón C, Scadding G, Timmermans M, Toskala E, Zhang N, Bousquet J. Uncontrolled allergic rhinitis and chronic rhinosinusitis: where do we stand today? Allergy 68: 1–7, 2013. doi: 10.1111/all.12040. [DOI] [PubMed] [Google Scholar]
- 17.Hilberg O, Pedersen OF. Acoustic rhinometry: recommendations for technical specifications and standard operating procedures. Rhinol Suppl 16: 3–17, 2000. [PubMed] [Google Scholar]
- 18.Ho ST, Hutmacher DW. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 27: 1362–1376, 2006. doi: 10.1016/j.biomaterials.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Holdsworth DW, Thornton MM. Micro-CT in small animal and specimen imaging. Trends Biotechnol 20: S34–S39, 2002. doi: 10.1016/S0167-7799(02)02004-8. [DOI] [Google Scholar]
- 19a.Hoxworth JM, Lal D, Fletcher GP, Patel AC, He M, Paden RG, Hara AK. Radiation dose reduction in paranasal sinus CT using model-based iterative reconstruction. AJNR Am J Neuroradiol 35: 644–649, 2014. doi: 10.3174/ajnr.A3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hupfer M, Nowak T, Brauweiler R, Eisa F, Kalender WA. Spectral optimization for micro-CT. Med Phys 39: 3229–3239, 2012. doi: 10.1118/1.4718575. [DOI] [PubMed] [Google Scholar]
- 21.Kelemen G. Nasal cavity of the guinea pig in experimental work. AMA Arch Otolaryngol 52: 579–596, 1950. doi: 10.1001/archotol.1950.00700030603006. [DOI] [PubMed] [Google Scholar]
- 22.Konradsen JR, Fujisawa T, van Hage M, Hedlin G, Hilger C, Kleine-Tebbe J, Matsui EC, Roberts G, Rönmark E, Platts-Mills TA. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J Allergy Clin Immunol 135: 616–625, 2015. doi: 10.1016/j.jaci.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M, Bonini S. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ 320: 412–417, 2000. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meltzer E, Ratner P, Bachert C, Carr W, Berger W, Canonica GW, Hadley J, Lieberman P, Hampel FC, Mullol J, Munzel U, Price D, Scadding G, Virchow JC, Wahn U, Murray R, Bousquet J. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol 161: 369–377, 2013. doi: 10.1159/000351404. [DOI] [PubMed] [Google Scholar]
- 24a.Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248: 254–263, 2008. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 25.Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: lessons from in vitro model systems and animal models. Eur Respir J 32: 487–502, 2008. doi: 10.1183/09031936.00023608. [DOI] [PubMed] [Google Scholar]
- 26.Moore BD, Hyde DM, Miller LA, Wong EM, Schelegle ES. Persistence of serotonergic enhancement of airway response in a model of childhood asthma. Am J Respir Cell Mol Biol 51: 77–85, 2014. doi: 10.1165/rcmb.2013-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabe T, Mizutani N, Shimizu K, Takenaka H, Kohno S. Development of pollen-induced allergic rhinitis with early and late phase nasal blockage in guinea pigs. Inflamm Res 47: 369–374, 1998. doi: 10.1007/s000110050346. [DOI] [PubMed] [Google Scholar]
- 28.Nathan RA, Eccles R, Howarth PH, Steinsvåg SK, Togias A. Objective monitoring of nasal patency and nasal physiology in rhinitis. J Allergy Clin Immunol 115, Suppl 1: S442–S459, 2005. doi: 10.1016/j.jaci.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obenaus A, Smith A. Radiation dose in rodent tissues during micro-CT imaging. J XRay Sci Technol 12: 241–249, 2004. [Google Scholar]
- 30.Ozdoganoglu T, Songu M. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis 6: 11–23, 2012. doi: 10.1177/1753465811431975. [DOI] [PubMed] [Google Scholar]
- 31.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature 484: 465–472, 2012. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkins CS, Fowler JF. A cautionary note on the resolution of paraquat lung damage after radiotherapy. Br J Radiol 58: 1137–1140, 1985. doi: 10.1259/0007-1285-58-695-1137. [DOI] [PubMed] [Google Scholar]
- 33.Phillips JE, Ji L, Rivelli MA, Chapman RW, Corboz MR. Three-dimensional analysis of rodent paranasal sinus cavities from X-ray computed tomography (CT) scans. Can J Vet Res 73: 205–211, 2009. [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips JE, Peng R, Harris P, Burns L, Renteria L, Lundblad LK, Fine JS, Bauer CM, Stevenson CS. House dust mite models: will they translate clinically as a superior model of asthma? J Allergy Clin Immunol 132: 242–244, 2013. doi: 10.1016/j.jaci.2012.12.1571. [DOI] [PubMed] [Google Scholar]
- 35.Rohrbach S, Olthoff A, Giefer B, Laskawi R, Götz W. Botulinum toxin type A induces apoptosis in nasal glands of guinea pigs. Ann Otol Rhinol Laryngol 110: 1045–1050, 2001. doi: 10.1177/000348940111001110. [DOI] [PubMed] [Google Scholar]
- 36.Roopa Manjunatha G, Roy Mahapatra D, Prakash S, Rajanna K. Validation of polyvinylidene fluoride nasal sensor to assess nasal obstruction in comparison with subjective technique. Am J Otolaryngol 36: 122–129, 2015. doi: 10.1016/j.amjoto.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Rüedi VL, Furrer W, Escher F, Lüthy F. Toxische wirkungen des streptomycins. Acta Otolaryngol 36, sup78: 66–77, 1948. doi: 10.3109/00016489.1948.11760817. [DOI] [Google Scholar]
- 38.Schladitz K. Quantitative micro-CT. J Microsc 243: 111–117, 2011. doi: 10.1111/j.1365-2818.2011.03513.x. [DOI] [PubMed] [Google Scholar]
- 39.Schreider JP, Hutchens JO. Morphology of the guinea pig respiratory tract. Anat Rec 196: 313–321, 1980. doi: 10.1002/ar.1091960307. [DOI] [PubMed] [Google Scholar]
- 40.Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr Opin Pharmacol 13: 351–361, 2013. doi: 10.1016/j.coph.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, Dawson DE, Dykewicz MS, Hackell JM, Han JK, Ishman SL, Krouse HJ, Malekzadeh S, Mims JW, Omole FS, Reddy WD, Wallace DV, Walsh SA, Warren BE, Wilson MN, Nnacheta LC; Guideline Otolaryngology Development Group. AAO-HNSF . Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg 152, Suppl: S1–S43, 2015. doi: 10.1177/0194599814561600. [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Wittmann HJ, Buschauer A, Schneider EH, Seifert R. Species-dependent activities of G-protein-coupled receptor ligands: lessons from histamine receptor orthologs. Trends Pharmacol Sci 34: 13–32, 2013. doi: 10.1016/j.tips.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Straszek SP, McLeod RL, Hey JA, Mosekilde L, Pedersen OF. Comparison of feline nasal cavity dimensions measured by acoustic rhinometry and nasal casts. Am J Rhinol 17: 233–239, 2003. doi: 10.1177/194589240301700410. [DOI] [PubMed] [Google Scholar]
- 44.Straszek SP, Pedersen OF. Nasal cavity dimensions in guinea pig and rat measured by acoustic rhinometry and fluid-displacement method. J Appl Physiol (1985) 96: 2109–2114, 2004. doi: 10.1152/japplphysiol.00540.2003. [DOI] [PubMed] [Google Scholar]
- 45.Straszek SP, Taagehøj F, Graff S, Pedersen OF. Acoustic rhinometry in dog and cat compared with a fluid-displacement method and magnetic resonance imaging. J Appl Physiol (1985) 95: 635–642, 2003. doi: 10.1152/japplphysiol.01105.2002. [DOI] [PubMed] [Google Scholar]
- 46.Thurmond R. Histamine in Inflammation. New York: Springer, 2011. doi: 10.1007/978-1-4419-8056-4. [DOI] [Google Scholar]
- 47.Thurmond RL. Histamine inflammation. Preface. Adv Exp Med Biol 709: vii–viii, 2010. [PubMed] [Google Scholar]
- 48.Vasquez SX, Shah N, Hoberman AM. Small animal imaging and examination by micro-CT. Methods Mol Biol 947: 223–231, 2013. doi: 10.1007/978-1-62703-131-8_18. [DOI] [PubMed] [Google Scholar]
- 49.Wang CY, Walfield AM, Fang X, Hammerberg B, Ye J, Li ML, Shen F, Shen M, Alexander V, MacGlashan DW. Synthetic IgE peptide vaccine for immunotherapy of allergy. Vaccine 21: 1580–1590, 2003. doi: 10.1016/S0264-410X(02)00732-6. [DOI] [PubMed] [Google Scholar]
- 50.Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med 372: 456–463, 2015. doi: 10.1056/NEJMcp1412282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White MV. The role of histamine in allergic diseases. J Allergy Clin Immunol 86: 599–605, 1990. doi: 10.1016/S0091-6749(05)80223-4. [DOI] [PubMed] [Google Scholar]
- 52.Widdicombe JG. Nasal pathophysiology. Respir Med 84, Suppl 1: 3–10, 1990. doi: 10.1016/S0954-6111(08)80001-7. [DOI] [PubMed] [Google Scholar]
- 53.Willekens I, Buls N, Lahoutte T, Baeyens L, Vanhove C, Caveliers V, Deklerck R, Bossuyt A, de Mey J. Evaluation of the radiation dose in micro-CT with optimization of the scan protocol. Contrast Media Mol Imaging 5: 201–207, 2010. doi: 10.1002/cmmi.394. [DOI] [PubMed] [Google Scholar]