Abstract
The retrotrapezoid nucleus (RTN) contains chemosensitive cells that distribute CO2-dependent excitatory drive to the respiratory network. This drive facilitates the function of the respiratory central pattern generator (rCPG) and increases sympathetic activity. It is also evidenced that during hypercapnia, the late-expiratory (late-E) oscillator in the parafacial respiratory group (pFRG) is activated and determines the emergence of active expiration. However, it remains unclear the microcircuitry responsible for the distribution of the excitatory signals to the pFRG and the rCPG in conditions of high CO2. Herein, we hypothesized that excitatory inputs from chemosensitive neurons in the RTN are necessary for the activation of late-E neurons in the pFRG. Using the decerebrated in situ rat preparation, we found that lesions of neurokinin-1 receptor-expressing neurons in the RTN region with substance P-saporin conjugate suppressed the late-E activity in abdominal nerves (AbNs) and sympathetic nerves (SNs) and attenuated the increase in phrenic nerve (PN) activity induced by hypercapnia. On the other hand, kynurenic acid (100 mM) injections in the pFRG eliminated the late-E activity in AbN and thoracic SN but did not modify PN response during hypercapnia. Iontophoretic injections of retrograde tracer into the pFRG of adult rats revealed labeled phox2b-expressing neurons within the RTN. Our findings are supported by mathematical modeling of chemosensitive and late-E populations within the RTN and pFRG regions as two separate but interacting populations in a way that the activation of the pFRG late-E neurons during hypercapnia require glutamatergic inputs from the RTN neurons that intrinsically detect changes in CO2/pH.
Keywords: abdominal activity, hypercapnia, sympathetic activity, ventilation, ventral medulla
INTRODUCTION
Breathing is generated and coordinated by excitatory and inhibitory circuits located in the brainstem (41, 42). Two oscillators are suggested to be responsible for respiratory rhythm and pattern generation (10). The first one is in the Bötzinger/pre-Bötzinger complexes (BötC/pre-BötC) of the ventral respiratory column (VRC), which is proposed to play a major role in inspiratory pattern generation (45, 46, 52). A second oscillator has been recently identified in the ventral medullary surface (VMS) and suggested to modify the respiratory pattern in states of elevated respiratory drive (16). This oscillator resides in the parafacial respiratory group (pFRG, also named as lateral parafacial), situated rostral to the VRC and ventrolateral to the facial nucleus, partially overlapped with the retrotrapezoid nucleus (RTN) chemoreceptor neurons located ventromedial to the facial nucleus (1, 14, 15).
In juvenile/adult animals, it has been described that the conditional expiratory oscillator in the pFRG is recruited mainly during metabolic challenges to increase pulmonary ventilation (17, 20, 26). At resting conditions, the expiratory neurons of the pFRG neurons are synaptically suppressed (9, 26, 38). In states of elevated inspired CO2 (hypercapnia) or low inspired O2 (hypoxia), rhythmic oscillations emerge in the pFRG, phase-locked to the late part of expiratory period (late-E), which are necessary to elicit contractions of the abdominal expiratory muscles through interactions with the VRC (1, 6, 32, 43, 44). In conditions of hypercapnia (29) or chronic hypoxia (30, 31, 56), the generation of late-E bursts in the abdominal motor activity is also associated with the occurrence of additional expiratory-related bursts in sympathetic nerve activity, suggesting that the presympathetic neurons of the ventral medulla may also receive excitatory drive from the pFRG expiratory oscillator (6, 29, 30).
Previous studies demonstrated that inhibition of the neurons of the VMS region that express the transcription factor phox2b eliminates the inspiratory and expiratory responses to hypercapnia (24, 44), raising the possibility that the pFRG expiratory neurons might also express phox2b and be intrinsically sensitive to CO2/H+. There is also evidence indicating that the activity of pFRG neurons is defined by synaptic inputs from other respiratory compartments, including the pre-BötC (15) and the dorsolateral pons (7, 18), as well as from peripheral chemoreceptors (6, 32) and pulmonary stretch receptors (20). Recent theoretical and experimental studies indicate that inhibitory synapses to the pFRG play an important role in the pattern formation of abdominal expiratory activity during hypercapnia (9, 18, 26, 43). With respect to excitatory neurotransmission, previous in silico studies predicted that CO2-dependent excitatory inputs from the RTN chemosensitive neurons could provide a mechanism for pFRG expiratory neurons to overcome the inhibition originating in the VRC (26). This observation agrees with studies suggesting that the neurons of the RTN and pFRG regions may be functionally distinct, although partially overlapped (15). However, there is no functional and anatomical evidence about the source and the involvement of excitatory neurotransmission in the pFRG for the recruitment of expiratory oscillator and the emergence of late-E bursts in the abdominal activity during hypercapnia. The investigation of these synaptic mechanisms is critical to understanding how the expiratory oscillator is physiologically engaged and determines the changes in the respiratory pattern.
Here, we investigated whether the RTN chemosensitive and the pFRG expiratory neurons may constitute functionally and phenotypically distinct but synaptically interacting populations. To check this hypothesis, we performed lesions of chemosensitive neurons of the RTN region using the saporin toxin conjugated with substance P (SSP-SAP) (48, 51) and evaluated the pattern of phrenic (PN), abdominal (AbN) and sympathetic nerve (SN) activities during hypercapnia. Also, to explore the contribution of excitatory neurotransmission to activate the expiratory neurons, we assessed the effects of the glutamatergic receptor antagonism in the pFRG region on the hypercapnia-induced inspiratory, expiratory, and sympathetic responses. Neuroanatomical studies were performed to gain evidence about the existence of direct projections from the RTN phox2b-expressing neurons to the pFRG region. Finally, we simulated the experimental data obtained in SSP-SAP-lesioned and glutamatergic receptor blockade conditions in a computational model to elucidate and promulgate this mechanism for excitatory control over the emergence of activity in the expiratory oscillator. Our data indicate that excitatory (glutamatergic) inputs from phox2b-expressing neurons of the RTN are necessary to stimulate the pFRG neurons in conditions of hypercapnia, but not during hypoxia, to generate late-E bursts in abdominal and sympathetic activities in rats.
MATERIALS AND METHODS
Animals.
Animal care and experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of São Paulo (protocol no. 07/2014) and São Paulo State University (protocol nos. 18/2014; 31/2016). All experiments were conducted using adult male Wistar rats weighing 270–300 g (in vivo anatomical experiments) or male Holtzman juvenile rats weighing 75–80 g (in situ experiments) at the time of surgery.
Immunotoxin lesions.
Surgical procedures were performed on juvenile rats anesthetized with an intraperitoneal injection of a mixture of ketamine and xylazine (100 and 7 mg/kg of body wt, respectively). Postsurgical protection against infection included intramuscular injections of two antibiotics (benzylpenicillin, 160,000 U/kg, and dihydrostreptomycin, 33.3 mg/kg). For selective chemical lesions of RTN chemoreceptor neurons, the rats were fixed to a stereotaxic frame and the coordinates used to locate the RTN (−2.1 and −2.3 mm from lambda; ± 1.6 mm from midline; −8.4 mm from the skull surface) were based on the stereotaxic atlas for rats (40). The tip of a pipette, connected to a Hamilton syringe, was inserted directly into the RTN for two bilateral injections of saporin conjugate [Sar9, Met (O2)11]-substance P (Advanced Targeting Systems, San Diego, CA) (0.6 ng in 100 nl of saline per side). Animals were allowed to survive 7–10 days before they were used for physiological experiments. The bilateral injections of toxin produced no observable behavioral effects, and these rats gained weight normally. Based on a previous publication (48, 51) and the present study, we did not notice any differences in neuroanatomical or physiological experiments in animals that received IgG-saporin or saline in the RTN region. Thus, in the present study, the sham-operated rats received injections of saline in the RTN (0.15 M, 100 nl per side). The dose of SSP-SAP used in the present study was selected based on previous experiments investigating the respiratory effects of SSP-SAP administration in the RTN region (37, 48, 51, 54). In agreement with our previous studies (48, 51), the selected dose created optimal lesion of the neurokinin-1 receptor (NK1R)-expressing neurons of the RTN while preserving the integrity of the cells that are immunoreactive to tyrosine hydroxylase (TH), choline acetyltransferase (ChAT), and tryptophan hydroxylase in the same region (data not shown in the present study).
In situ working heart-brainstem preparation.
Naïve rats, or rats that received injections of SSP-SAP in the RTN (7–10 days before the experiments), were surgically prepared to obtain the in situ working heart-brainstem preparations, as previously described (39, 56). The animals were initially anesthetized with halothane (AstraZeneca, São Paulo, Brazil) until the loss of the paw withdrawal reflex, transected caudal to the diaphragm, submerged in a chilled Ringer solution, comprised of (in mM) 125 NaCl, 24 NaHCO3, 3 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, and 10 dextrose, and decerebrated at the precollicular level. In experiments involving injections of kynurenic acid (Kyn) in the pFRG (see below), the preparations were placed supine and the head fixed for exposure of the ventral surface of the medulla. To this, the trachea, esophagus, and all muscles and connective tissues covering the basilar surface of the occipital bone were removed, the basilar portion of the atlanto-occipital membrane was cut, and the bone was carefully removed using a micro-Rongeur (D.L. Micof, São Paulo, Brazil). Lungs were removed and then the preparations were transferred to a recording chamber. The descending aorta was cannulated and perfused retrogradely using a roller pump (Watson-Marlow 502s, Cornwall, UK) via a double-lumen cannula. The perfusate consisted of Ringer solution containing 1.25% polyethyleneglycol (an oncotic agent, Sigma, St. Louis, MO) and neuromuscular blocker (vecuronium bromide, 3–4 μg/ml, Cristália Produtos Químicos Farmacêuticos, São Paulo, Brazil). The solution was gassed continuously with 5% CO2-95% O2, warmed to 31–32°C, and filtered using a nylon mesh (pore size 25 μm, Millipore, Billirica, MA). The perfusion pressure was maintained in the range of 50–70 mmHg by adjusting the rate flow to 21–25 ml/min and by adding vasopressin to the perfusate (0.6–1.2 nM, Sigma).
Nerve recording and data analyses.
PN, AbN, and SN activities were recorded simultaneously using bipolar glass suction electrodes held in micromanipulators (Narishige, Tokyo, Japan). Left PN activity was recorded from its central end, and its rhythmic ramping activity was used to monitor preparation viability. An AbN was isolated from the abdominal muscles on the right at thoracic–lumbar level (T12-L1), cut distally, and recorded. The sympathetic activity was recorded from the thoracic SN (tSN) chain at the level of T10–T12. The signals were amplified, band-pass filtered (0.1–3 kHz; Grass Technologies, Middleton, WI), and acquired with an analog-to-digital converter (CED 1401; Cambridge Electronic Design, Cambridge, UK) to a computer using Spike2 software (version 7; Cambridge Electronic Design) at a sampling rate of 5 kHz. At the end of the experiments, the perfusion pump was turned off to determine the electrical noise (after the death of the preparations).
The analyses were carried out on rectified and smoothed signals (time constant of 50 ms) and performed offline using Spike 2 software (version 7.1, CED) after noise subtraction. PN activity was evaluated by its burst frequency (burst per minute), amplitude (µV), duration (time of inspiration, s), and time interval (time of expiration, s). AbN and tSN activities were determined as mean values of integrated signals (µV). Additionally, phrenic-triggered averages of AbN and tSN were generated from 1 to 3 min epochs for analyses of burst pattern across the respiratory cycle. In these averaged signals, the respiratory cycle was divided into inspiration (insp, coincident with PN burst of raw signal), postinspiration (post-I, initial 2/3 of expiratory phase), and late-E (reaming 1/3 of expiratory phase). Mean AbN activity was calculated during late-E phase, whereas mean tSN activity was determined in each respiratory phase. Additionally, the amplitude of respiratory-related burst in tSN was calculated as the value difference (µV) between the lower “tonic” activity during expiration and the peak activity during late-inspiration/beginning of postinspiration. Baseline PN, AbN, and tSN activities were expressed in raw units. The maximal changes in the PN amplitude and AbN and tSN mean activities induced by hypercapnia (see below) were calculated as percentage values in relation to basal values immediately before the stimulus. The changes in tSN and AbN activities elicited by KCN were calculated as the percentage variations of the area under the curve in relation to basal activities before stimulus. The changes in the PN burst frequency and the time of inspiration and expiration were expressed in their raw units. The perfusion pressure was also evaluated as the maximal variation during stimulus administration and expressed as mmHg.
Hypercapnia and stimulation of peripheral chemoreceptors.
After stabilization and initial baseline recordings, the in situ preparations were exposed to hypercapnia by raising the fractional concentration of CO2 from 5 to 8 or 10% (balanced with O2) in the perfusate. In the experiments with SSP-SAP rats, CO2 was increased to 10% for 5 min, whereas for experiments with injections in the pFRG of naïve rats (see below), CO2 was increased to 8% for 8–10 min. The rationale to use a lower level of CO2 in the latter group was to prevent possible problems resulting from prolonged exposures (>5 min) to high CO2/low pH. The hypercapnic effects peaked and reached steady-state approximately after 3 min of exposure. The peripheral chemoreceptors were acutely stimulated in in situ preparations of control and SSP-SAP rats with injections of KCN (0.05%, 50 µl) into the descending aorta via the perfusion cannula, as previously described (8, 32).
Intraparenchymal injections in situ.
The broad spectrum ionotropic glutamatergic antagonist Kyn (100 mM in sterile saline pH 7.4) was injected (Picospritzer III, Parker Hannifin) bilaterally in the pFRG of in situ preparations through single-barrel glass pipettes (20-µm tip diameter). Injections were made according to the following coordinates: 0.5 mm caudal from trapezoid body, 1.7–1.8 mm lateral to midline, and 50 µm beneath the ventral surface. Injections were performed during hypercapnia (during steady state), and the effects were monitored for at least 5 min. The volume of each injection was 20–30 nl, and the time apart of each injection was less than 60 s. Injection sites were confirmed post hoc by the verification of the track of the pipette in the pFRG region. A second hypercapnic stimulation was applied 60 min after Kyn injections to verify the drug washout and the recovery of the responses.
Anatomical experiments.
Tracer injections were made in adult rats anesthetized with a mixture of ketamine and xylazine (100 mg/kg ip and 7 mg/kg ip, respectively). The surgery used standard aseptic methods, and after that surgery, the rats were treated with the antibiotic ampicillin (100 mg/kg im) and analgesic ketorolac (0.6 mg/kg sc). A group of three rats received iontophoresis injections (5-μA positive current pulses, 7-s duration every 7 s for 10 min) of the retrograde tracer cholera toxin subunit b (CTb) (low salt, 1% in distilled water, List Biological, Campbell, CA) into the lateral aspect of ventral surface, reaching pFRG region, using a glass micropipette with an internal tip diameter of 20 μm. These injections were made using the following coordinates: 2.6–2.8 mm caudal to lambda, 2.6–2.7 mm lateral to midline, and 8.5–8.6 mm below the dorsal surface of the brain., the animals were anesthetized and immediately perfusion-fixed, as described below, 7 to 10 days after the CTb injection.
Histology.
The rats were deeply anesthetized with pentobarbital sodium (60 mg/kg ip), injected with heparin (500 U intracardially), and finally perfused through the ascending aorta initially with 250 ml of phosphate-buffered saline (pH 7.4) and then with 500 ml of 4% phosphate-buffered paraformaldehyde (0.1 M, pH 7.4). The brains were extracted, cryoprotected by overnight immersion in a 20% sucrose solution in phosphate-buffered saline at 4°C, sectioned in the coronal plane at 40 μm on a sliding microtome, and stored in cryoprotectant solution (20% glycerol plus 30% ethylene glycol in 50 mM phosphate buffer, pH 7.4) at −20°C for up to 2 wk awaiting histological processing. All histochemical procedures were done using free-floating sections, according to previously described protocols (4, 48).
The substance-P receptor (NK1R) was detected using a rabbit anti-NK1R antibody (1:5,000, Sigma) raised against a synthetic peptide corresponding to the C-terminal of NK1R of rat origin (amino acids 393–407) followed by Alexa 488-donkey anti-rabbit (1:200, Jackson Laboratories, West Grove, PA). Phox2b was detected using a rabbit anti-phox2b antibody (1:800, gift from J.-F. Brunet, Ecole Normale Supèrieure, Paris, France) followed by Cy3 donkey anti-rabbit IgG (1:200, Jackson). TH was detected using mouse anti-TH (MAB318, 1:1,000; Millipore, Temecula, CA), followed by donkey anti-mouse Alexa488 IgG (1:200; Jackson). ChAT was detected using goat anti-ChAT (AB144P, 1:200; Millipore) followed by mouse anti-goat Alexa488 IgG (1:200; Invitrogen, Carlsbad, CA). CTb was detected using a goat anti-CTb antibody (1:2,000, List Biological) followed by Alexa 488 donkey anti-goat IgG (1:200, Jackson). The specificity of the antibodies has been validated previously (5, 51, 53).
Cell mapping, counting, and imaging.
A conventional multifunction Zeiss Axioskop 2 microscope (Oberkochen, Germany) was used for all observations. ImageJ software (https://imagej.nih.gov/ij/) was used to count the various types of neuronal and receptors profile within a defined area and merge the color channels in photographs in the dual-labeling experiments. Section alignment between brains was done relative to a reference section. To align sections around the RTN/pFRG and the raphe pallidus/parapyramidal region level, the most caudal section containing an identifiable cluster of facial motor neurons was identified in each brain and assigned the level 11.6 mm caudal to bregma (40). Levels rostral or caudal to this reference section were determined by adding a distance corresponding to the interval between sections multiplied by the number of intervening sections. It was analyzed seven sections to the RTN/pFRG, seven sections to C1 region, five sections to facial nucleus, nine sections to dorsal motor nucleus of the vagus, and eight sections to nucleus ambiguus. The same method was also used to identify the bregma level of the BötC, the pre-BötC, and the rostral ventral respiratory group (rVRG). The NK1R-ir was quantified by densitometric analysis. To this, images were taken through the region of interest from both sides of the brainstem using the same exposure time. The area of interest was outlined using the landmarks as follows. The RTN region (bregma at −11.6 mm) was defined by outlining from halfway down the medial edge of the facial motor nucleus around the center of the ventral edge of this nucleus and then perpendicular to the ventral surface. The region continued medially along the ventral surface to the medial edge of the pyramidal tract and closed with a diagonal to the medial edge of the facial nucleus. Note that this region of the brain thus defined encompassed not only the RTN but a large region medial to it. This choice was made because RTN per se is not identifiable in tissue reacted for standard NK1R immunochemistry because of the extreme low level of immunoreactivity associated with the Phox2b-expressing neurons (35, 48, 51). Bötzinger region (bregma at −11.8 mm), pre-Bötzinger region (bregma at −12.6 mm), and rVRG (bregma at −13.3 mm) were defined by an oval area 350 μm wide and 500 μm long, with the top centered on the ventral edge of the ambiguus. Using ImageJ software, the region was outlined, and the region of interest was segmented such that the segments were judged to represent true immunostaining using the nucleus ambiguus as a standard between sections. The area of pixels containing segments was calculated by the software, and the data are represented as percentage of control, with control as 100%. All files were exported to the Canvas 9 software-drawing program for final modifications. Photographs were taken with a 12-bit color charge-coupled device camera (CoolSnap, Roper Scientific, Tuscon, AZ; resolution 1,392 × 1,042 pixels).
Computational model.
The computational model used in this study was adapted from the model presented in Barnett et al. (6), including the presympathetic circuitry described in Baekey et al. (3) and Molkov et al. (29). These models are ultimately based on the functional spatial architecture of the respiratory pattern generator originally published by Smith et al. (45) and recently reviewed by Molkov et al. (27), where the neurons were modeled using a Hodgkin-Huxley formalism. In this computational framework, the respiratory rhythm is organized by three principle inhibitory populations [the early-inspiratory population (early I) in the pre-BötC, the postinspiratory population (post-I) in the BötC, and the augmenting-expiratory population (aug-E) in the BötC] and the excitatory preinspiratory/inspiratory population (pre-I/I) in the pre-BötC. In addition to the respiratory central pattern generator (rCPG), this model includes the expiratory oscillator of the pFRG and presympathetic circuitry in the rostral ventrolateral medulla (RVLM), which integrates phase-spanning input from the pons and the medullary respiratory circuits as well as late-E input from the conditional pFRG expiratory oscillator. In this model, the RVLM was considered to provide the pattern for the sympathetic motor output (description in Refs. 3 and 29). The excitatory drives and the corresponding source populations are indicated in Table 1. We identified key model parameters to describe the mechanisms that we attributed to the decrease in inspiratory activity and suppression of active expiration during hypercapnia in the SSP-SAP lesion experiments and the suppression of active expiration in Kyn injection experiments.
Table 1.
The excitatory drives and the corresponding source populations considered in the model
| Target Population | Excitatory Drive [Synaptic Weight] or Source Population [weight of synaptic input from single neuron] |
|---|---|
| IE (pons) | post-I [0.35]; ramp-I [0.2] |
| RVLM | Drive VLM [1]; IE [0.05]; early-I (2) [−0.01]; late-E [0.03]a; post-I [−0.05] |
| aug-E (BötC) | Drive pons [1.2]b; drive RTN [1.5]b,c; early-I (1) [−0.135]; late-E [0.03]; post-I [−0.3] |
| early-I (1) (pre-BötC) | Drive pons [0.6]b; drive RTN [1.5]b,c; drive raphe [0.5]; aug-E [−0.265]; post-I [−0.45]; pre-I/I [0.05] |
| early-I (2) (rVRG) | Drive pons [2.5]; aug-E [−0.25]; post-I [−0.75]b |
| late-E (pFRG) | Drive RTN [0.24]b,c; early-I (1) [−0.05]; post-I [−0.0275]; pre-I/I [0.013]; late-E [0.024] |
| post-I (BötC) | Drive pons [1.45]b; early-I (1) [−0.025] |
| post-I (e) (BötC) | Drive pons [0.8]; aug-E [−0.3]; early-I (1) [−0.2] |
| pre-I/I (pre-BötC) | Drive RTN [0.22]b; drive pons [0.65]; drive raphe [0.15]; aug-E [−0.01]; post-I [−0.19]; pre-I/I [0.02] |
| ramp-I (rVRG) | Drive pons [2]; aug-E [−0.1]; early-I (2) [−0.3]; post-I [−2]; pre-I/I [0.12] |
aug-E, augmenting-expiratory population; BötC, Bötzinger complex; IE, inspiratory-expiratory; pFRG, parafacial respiratory group; post-I, postinspiration; pre-I/I, preinspiratory/inspiratory; RTN, retrotrapezoid nucleus; rostral ventrolateral medulla, RVLM; rVRG, rostral ventral respiratory group.
Not present in Baekey et al. (3);
different from Barnett et al. (7);
must be multiplied by depending on CO2 partial pressure (see methods).
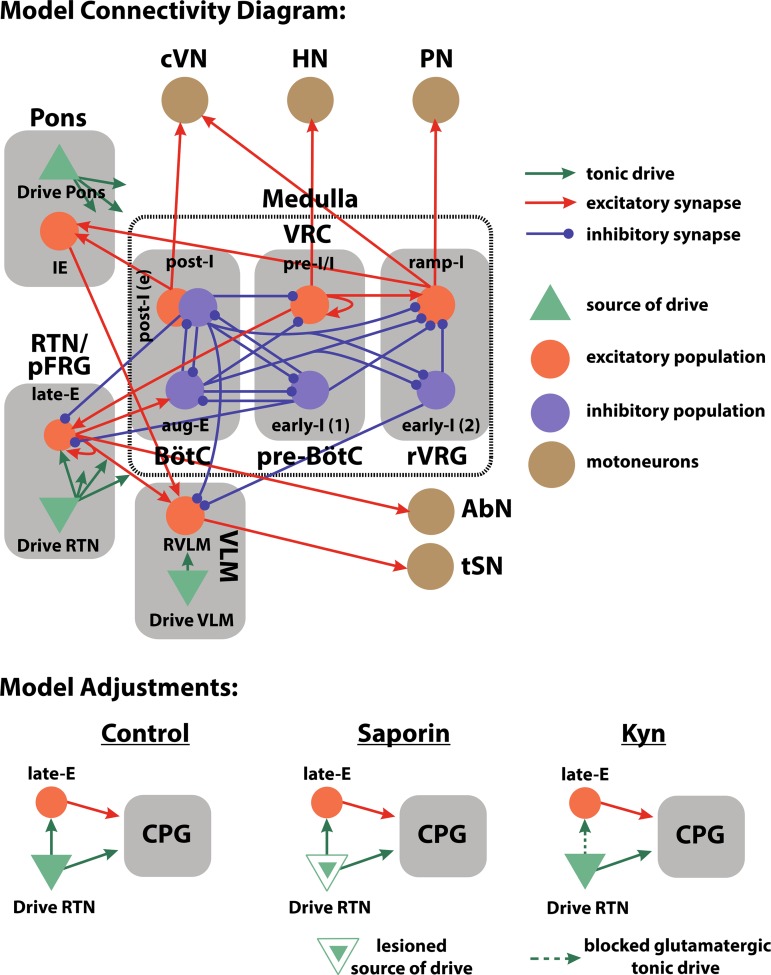
The RTN chemosensory neuron population projects into the rCPG, and it is thought to provide a critical component of tonic drive to the inspiratory and the E2 phase neurons of the CPG. In the present model, the CO2-sensitive RTN drive projects to the pre-I/I (pre-BötC), early-I (1) (pre-BötC), and aug-E (BötC) populations. We implemented modulation of the RTN drive by CO2 percentage by multiplying the RTN input by the factor f(CO2) = 1.3α tanh(CO2/7.2), where the coefficient α takes value 0.5 during simulations of the SSP-SAP condition and 1 otherwise. Unless noted, simulations of eucapnia take place at 5% CO2, and simulations of hypercapnia take place at 10% CO2. We implemented Kyn injections in the model as the removal of the excitatory projection from the RTN drive to late-E (pFRG) (Fig. 10). This change does not impact simulations during eucapnia since the late-E (pFRG) population is not active during eucapnia.
Fig. 10.
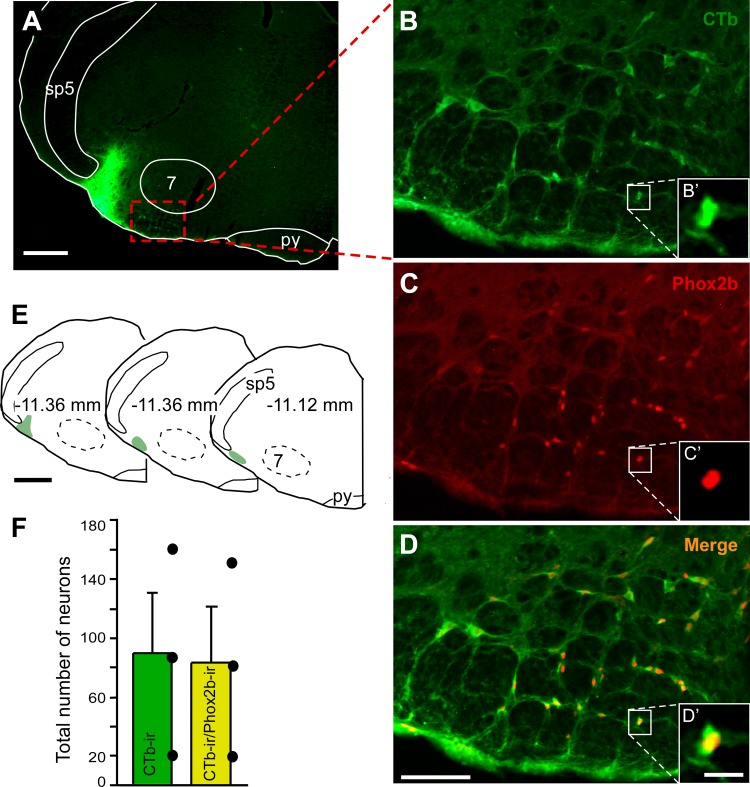
Projections from retrotrapezoid nucleus (RTN) to parafacial respiratory group (pFRG) revealed by retrograde tracer. A: photomicrographies showing the typical injection site of the retrograde tracer cholera toxin subunit b (CTb) in the pFRG region. B–D: examples of neurons retrogradely labeled from the pFRG (CTb, B) and Phox2b-immunoreactivity (C) located in the RTN and colocalized (D). B′–D′: higher magnification of the region outlined in B–D. E: computer-generated plot of three injections of CTb that were confined to the pFRG, bregma level −11.36 to −11.12 mm, according to Paxinos and Watson (40). F: total number of RTN neurons (n = 3) detected in seven sections per brain (CTb and CTb + Phox2b neurons). Scales bar = 0.5 mm in A, 50 μm in D, 10 μm in D′, and 1 mm in E. 7, facial motor nucleus; py, pyramide; sp5, spinal trigeminal tract.
Statistics.
Statistical analyses were performed using Sigma Stat (version 3.0; Jandel Corporation, Point Richmond, CA). Data are reported as means ± SE. The normal distribution of the data was verified with Shapiro-Wilk normality test. Changes in baseline activity elicited by SSP-SAP lesions or Kyn injections in the RTN or pFRG regions were compared using unpaired and paired Student's t-tests, respectively. Effects of SSP-SAP or Kyn injections on the responses to hypercapnia were compared using two-way ANOVA and repeated-measure one-way ANOVA, respectively, followed by Newman-Keuls posttest. Cell counting was analyzed using one-way ANOVA, followed by Newman-Keuls posttest. Significance was set at P ≤ 0.05. Graphs were generated using GraphPad Prism (version 6, La Jolla, CA).
RESULTS
Substance P saporin toxin selectively destroys the chemically coded neurons of the RTN region.
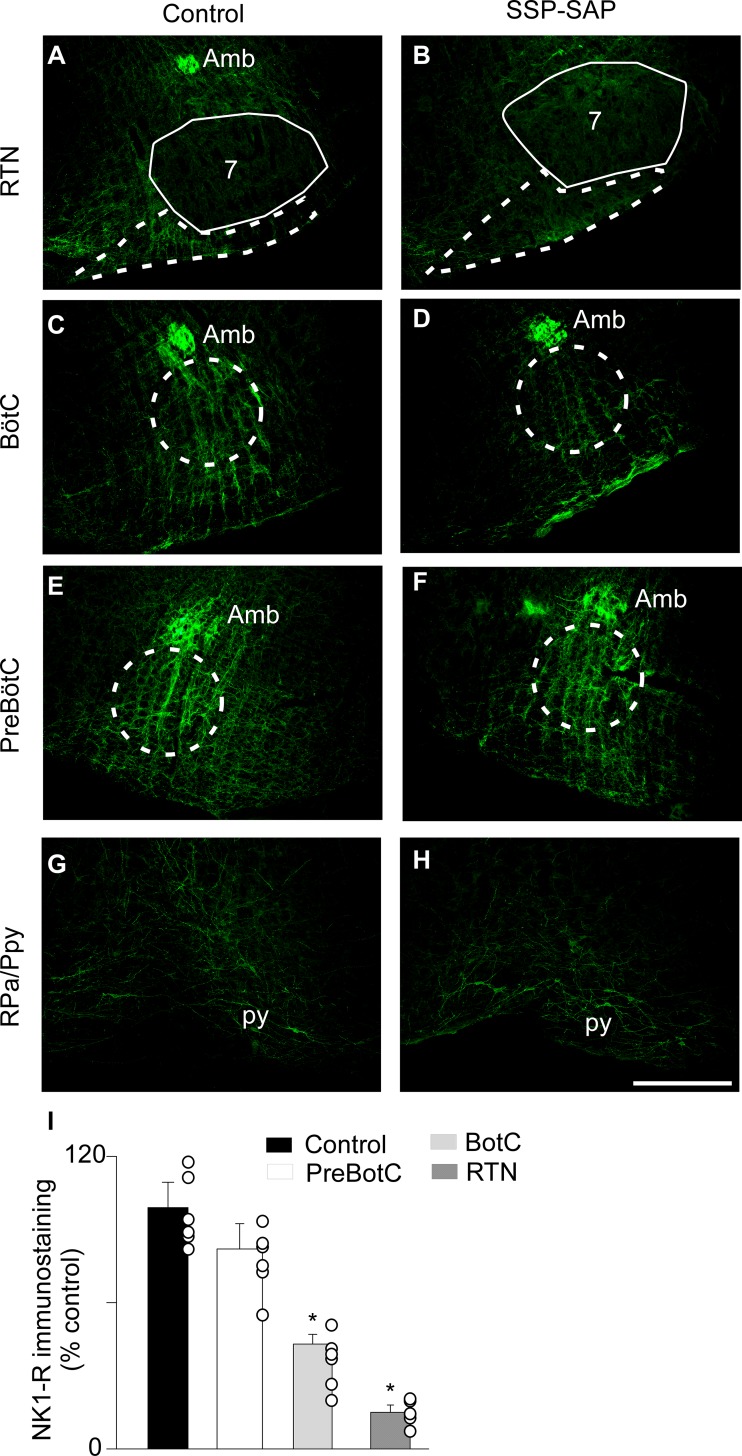
Substance P-immunoreactive receptors were abundant in the region of RTN (Fig. 1A), in agreement with previous studies (37, 48, 51). After bilateral injections (7–10 days) of SSP-SAP (0.6 ng/100 nl) into the RTN (n = 6), we evaluated the intensity of the NK1R-ir within the VMS as well as in 3 consecutive segments of the VRC: the BötC, the pre-BötC, and the rVRG. The 0.6 ng/site dose of SSP-SAP markedly reduced the NK1R-ir within the RTN region, as well as medial to it, i.e., at raphe pallidus/parapyramidal [Fig. 1, A, B, and G–I; lesion side: 15 ± 6% of control animals (n = 6); P = 0.032]. A small but significant reduction was also noted within the BötC, the region of the VRC closest to the RTN (Fig. 1, C, D, and I; lesion side: 43 ± 4% of control animals; P = 0.041), compared with controls. No changes of NK1R-ir were detected further caudally within the VRC [Fig. 1, E, F, and I; pre-BötC: 82 ± 11% of control animals (P = 0.0845); rVRG lesion side: 99 ± 6% of control animals (P = 0.32)].
Fig. 1.
Selectivity of the lesions induced by saporin-substance P conjugate (SSP-SAP) injection within the retrotrapezoid nucleus (RTN) region. Representative photomicrographs of rats treated with saline (control; A, C, E, and G) or SSP-SAP (B, D, F, and H). Neurokinin-1 receptor (NK1R)-immunoreactivity (ir) at the RTN (A and B), BötC (C and D), pre-BötC (E and F), and raphe pallidus/parapyramidal (RPa/Ppy) (G and H) levels. Analysis of the extent of NK1R-ir (I) in the regions observed in A–F. NK1R-ir in SSP-SAP group is expressed as a percentage of the immunoreactivity observed in the control group. Scale bar = 0.5 mm. *Different from control group, P < 0.05. 7, facial motor nucleus; Amb, nucleus ambigus; BötC, Bötzinger complex; py, pyramide tract.
To further assess the selectivity of the lesions, we analyzed Phox2b/TH-ir and TH-ir within the ventrolateral medulla of SSP-SAP-injected rats. Figure 2 is a representative example from a rat that had received a bilateral injection of 0.6-ng dose of SSP-SAP within the RTN region. Phox2b+/TH− neurons were considerably reduced by 63 ± 0.06%, whereas no significant changes were found in the number of TH+ neurons (Fig. 2, A–G). We also noted that the fraction of catecholaminergic neurons that expressed detectable levels of Phox2b was also unaffected by the toxin (Fig. 2F). This observation indicates that SSP-SAP selectively killed the Phox2b+/TH− neurons and the resistance of TH+ neurons to SSP-SAP is consistent with previous observations (12, 51, 54).
Fig. 2.
Substance P-saporin conjugate (SSP-SAP) in the retrotrapezoid nucleus (RTN) reduces phox2b+/tyrosine hydroxylase (TH)− neurons. Representative photomicrographs of the RTN of rats treated with saline (control; A–C) or SSP-SAP (D–F). In A and D, phox2b-immunoreactivity nuclei appear in red (Cy3), in B and E, TH appears in green (Alexa 488), and in C and F, merge is shown in yellow. Group data (G). *Different from control group, P < 0.05. Scale bar = 100 µm. 7, facial motor nucleus.
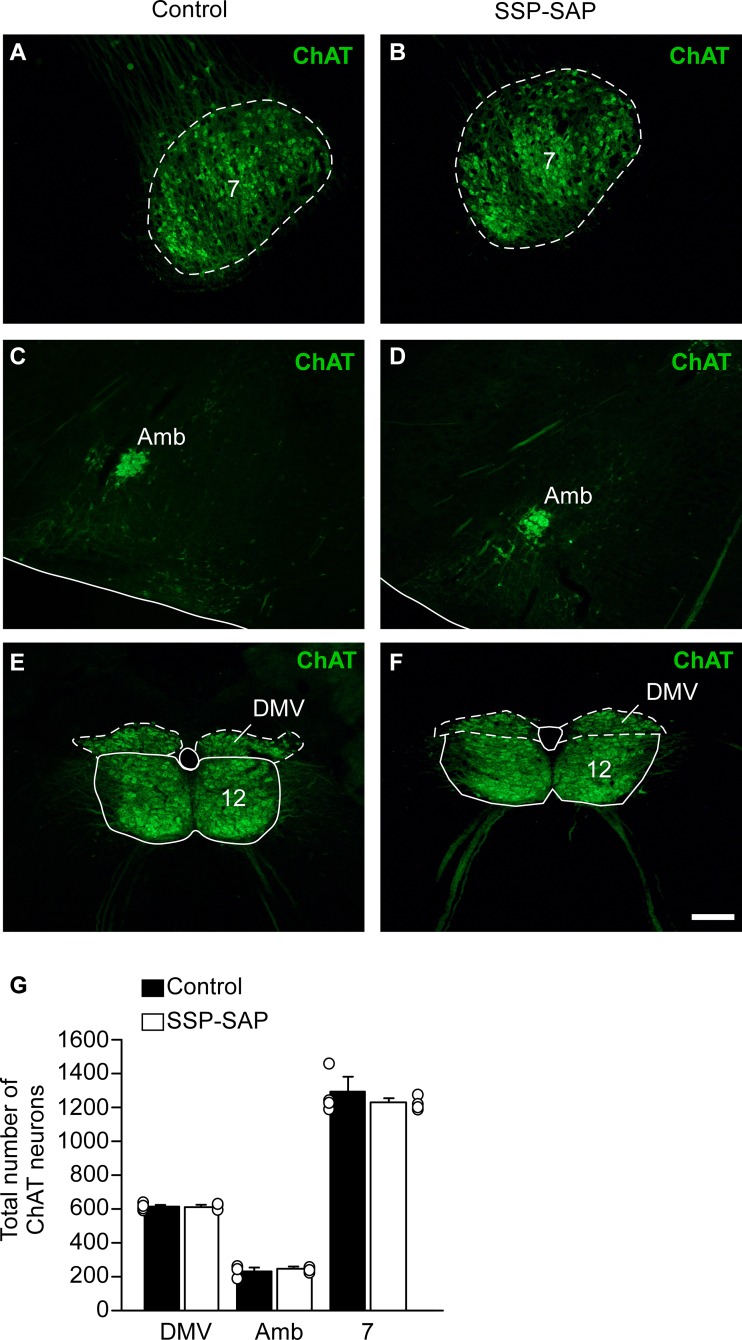
We also systematically examined the effect of SSP-SAP on specific types of neurons located in the immediate proximity of RTN. Based on ChAT immunoreactivity, the toxin had no detectable effect on facial motor neurons (Fig. 3, A, B, and G). The nucleus ambiguus, dorsal motor nucleus of the vagus and the hipoglossal nucleus were also spared, presumably because of its physical distance from the SSP-SAP injection site rather than by a lack of sensitivity to the toxin, given that the nucleus ambiguus expresses extremely high levels of NK1Rs (Fig. 3, C–G).
Fig. 3.
Selectivity of the lesions induced by substance P-saporin conjugate (SSP-SAP) in the retrotrapezoid nucleus (RTN) region. Choline acetyl-transferase immunoreactivity (ChAT-ir) of rats treated with saline (control; A, C, and E) or SSP-SAP (B, D, and F) at facial motor nucleus (7) (A and B), nucleus ambiguus (Amb) (C and D), or dorsal motor nucleus of the vagus (DMV) and hypoglossal motor nucleus (12) (E and F). Note that cholinergic neurons seem unaffected by the toxin. Group data (G). Each column represents the total number of cholinergic neurons. Scale bar = 100 µm.
Sympathetic and respiratory activities in SSP-SAP-lesioned rats at rest, under hypercapnia, and during peripheral chemoreceptor stimulation.
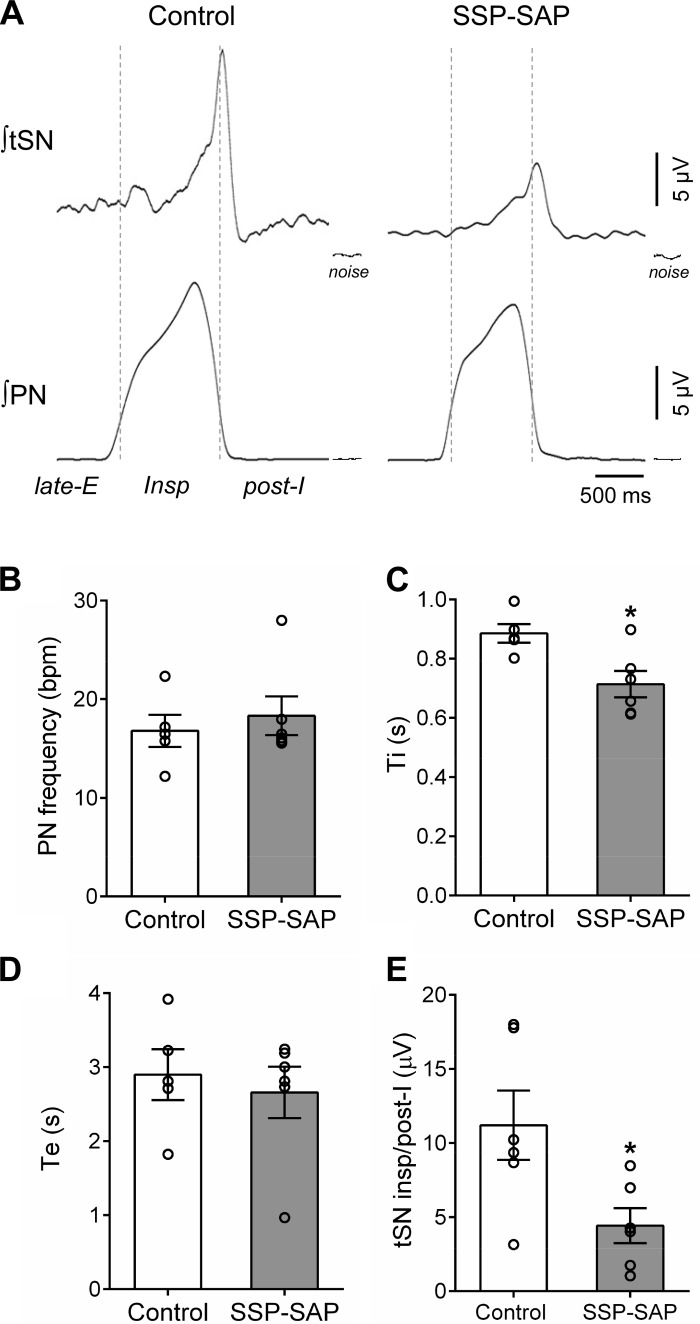
In this series of experiments, we investigated the effects of bilateral lesions of the NK1-expressing neurons of the RTN region with SSP-SAP on the respiratory and sympathetic activities under eucapnia (baseline), during hypercapnia (10% CO2), and during the stimulation of peripheral chemoreceptors with injections of KCN in the in situ preparations. At baseline conditions (Fig. 4A), preparations of SSP-SAP-lesioned rats (n = 6) showed, compared with controls (n = 6): 1) similar PN burst amplitude (16.2 ± 3.8 vs. saline: 14.1 ± 3.1 µV) and frequency (20 ± 3 vs. control: 17 ± 2 burst/min; Fig. 4B) and 2) reduced inspiratory time (0.714 ± 0.044 vs. control: 0.885 ± 0.031 s; P = 0.0145; Fig. 4C) and similar expiratory time (2.66 ± 0.35 vs. control: 2.90 ± 0.34 s; Fig. 4D). With respect to the sympathetic activity, both groups of in situ preparations showed respiratory-related bursts in tSN that peaked during late-inspiration/beginning of postinspiration (Fig. 4A). However, the magnitude of inspiratory/postinspiratory burst was smaller in SSP-SAP-lesioned rats compared with controls (4.4 ± 1.2 vs. control: 11.2 ± 2.3 μV; P = 0.0273; Fig. 4E). The in situ preparations with lesions of NK1-positive neurons in the RTN region also exhibited lower levels of mean tSN during post-I (5.2 ± 1.3 vs. saline: 10.34 ± 2.0 µV; P = 0.05) and late-E phases (4.9 ± 1.0 vs. saline: 8.5 ± 1.2; P = 0.05).
Fig. 4.
Baseline respiratory and sympathetic activities of in situ rat preparations with lesions of neurokinin-1 receptor-expressing neurons in the retrotrapezoid nucleus (RTN). A: cycle-triggered averages of integrated (∫) phrenic nerve (PN) and thoracic sympathetic nerve (tSN) activities of representative preparations that received injections of saline (control) or substance P-saporin conjugate (SSP-SAP). The dotted lines delineate the respiratory phases: inspiration (insp), postinspiration (post-I), and late-expiration (late-E). B–E: average values of PN burst frequency, times of inspiration (Ti) and expiration (Te), and respiratory-related burst of tSN observed during insp/post-I phases, respectively, of control (n = 6) and SSP-SAP groups (n = 6). *Different from control group, P < 0.05.
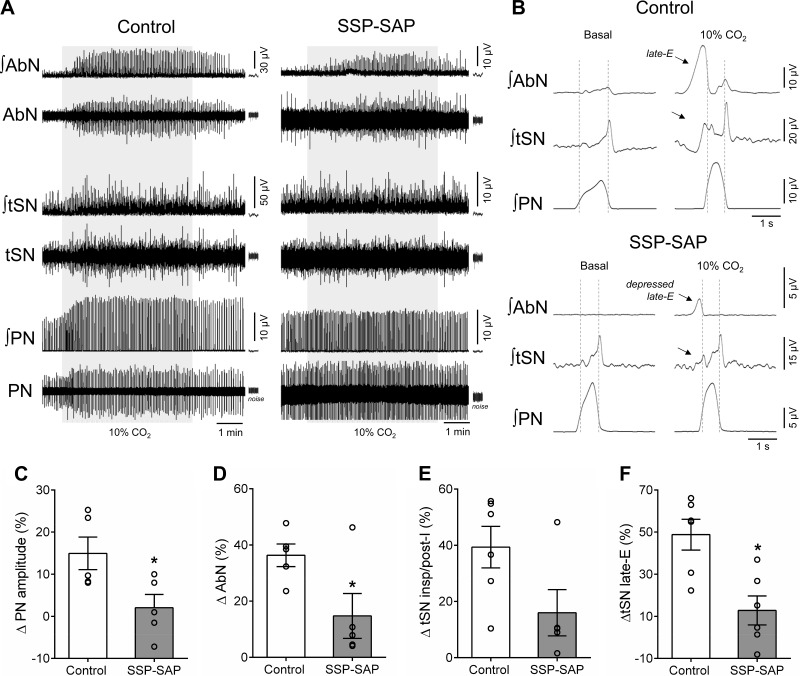
Under hypercapnia, the PN burst amplitude, but not the frequency, of in situ preparations of the control group increased in association with the emergence of high-amplitude bursts in the AbN activity during late-E phase (Fig. 5, A–D; P < 0.05). Hypercapnia also elevated the tSN levels during inspiration/postinspiration as well as brought about novel bursts during late-E phase, associated with the occurrence of late-E bursts in AbN (Fig. 5, B, E, and F; P < 0.01). In the SSP-SAP group, the respiratory and sympathetic reflex responses to hypercapnia were remarkably depressed. Specifically, we verified that 1) the increase in the PN burst amplitude was abolished (∆ = 2 ± 3% vs. control: 15 ± 4%; P = 0.0325; Fig. 5, B and C), with no significant changes in PN burst frequency (∆ = −2 ± 2 vs. control: 3 ± 2 burst/min), 2) the amplitude of AbN late-E bursts decreased (∆ = 15 ± 8% vs. control: 36 ± 4%; P = 0.04; Fig. 5, B and D), 3) the increase in tSN during inspiration/postinspiration was smaller, albeit not statistically significant (∆ = 16 ± 8% vs. control: 39 ± 7%; P = 0.0572; Fig. 5, B and E), and 4) the increase in tSN activity during late-E was attenuated (∆ = 13 ± 7% vs. control: 49 ± 7%; P = 0.05; Fig. 5, B and F).
Fig. 5.
Respiratory and sympathetic reflex responses to hypercapnia of in situ rat preparations with lesions of neurokinin-1 receptor-expressing neurons in the retrotrapezoid nucleus (RTN). A: raw and integrated (∫) recordings of the abdominal nerve (AbN), thoracic sympathetic nerve (tSN), and phrenic nerve (PN) from representative preparations of control and saporin-substance P conjugate (SSP-SAP) groups, illustrating the responses to hypercapnia (10% CO2 for 5 min, gray box). Note that the scales for AbN and tSN recordings from control and SSP-SAP in situ preparations are different. B: cycle-triggered averages of integrated AbN, tSN, and PN activities of representative preparations of control and SSP-SAP groups, showing the pattern of activities during eucapnia (basal) and hypercapnia (10% CO2). Note that hypercapnia evoked late-expiration (late-E) bursts in AbN and tSN in the control preparation, which is markedly depressed in SSP-SAP preparation (arrows). C–F: average variations (∆) of PN burst amplitude, AbN activity during late-E phase, tSN during inspiration/postinspiration, and late-E, respectively, of control (n = 6) and SSP-SAP groups (n = 6). *Different from control group, P < 0.05.
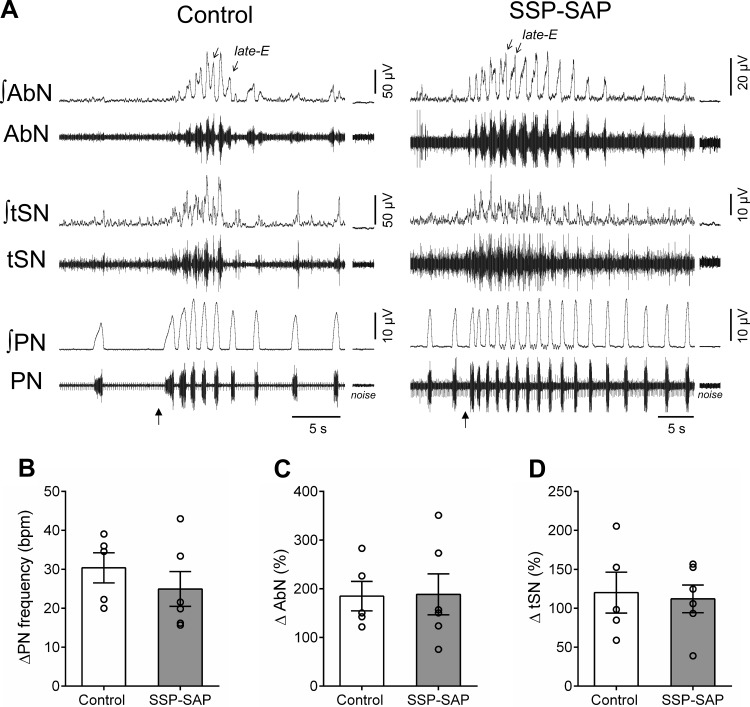
With respect to peripheral chemoreflex stimulation, injections of KCN accelerated PN burst frequency and increased AbN and tSN activities of in situ preparations of control and SSP-SAP rats (Fig. 6A). The magnitude of PN frequency (∆ = 30 ± 4 vs. 25 ± 4 burst/min, Fig. 6B), AbN (∆ = 185 ± 30% vs. 189 ± 42%, Fig. 6C), and tSN responses (∆ = 120 ± 26% vs. 112 ± 18%, Fig. 6D) was similar between groups. Noteworthy, during peripheral chemoreceptor stimulation, late-E bursts were observed in the AbN activity of SSP-SAP group (Fig. 6A) but were attenuated during the exposure to hypercapnia (Fig. 5, B and D).
Fig. 6.
Respiratory and sympathetic reflex responses to stimulation of peripheral chemoreceptors of in situ rat preparations with lesions of neurokinin-1 receptor-expressing neurons in the retrotrapezoid nucleus (RTN). A: raw and integrated (∫) recordings of the abdominal nerve (AbN), thoracic sympathetic nerve (tSN), and phrenic nerve (PN) from representative preparations of control and saporin-substance P conjugate (SSP-SAP) groups, illustrating the transient responses to peripheral chemoreceptor stimulation (KCN, 0.05%, arrows). Note the presence of late-expiration (late-E) bursts in AbN activity of control and SSP-SAP preparations during peripheral chemoreceptor activation. B–D: average variations (∆) of PN burst frequency and mean AbN and tSN activities, respectively, of control (n = 6) and SSP-SAP groups (n = 6).
Effects of bilateral injection of Kyn in the pFRG on respiratory and sympathetic outflows.
The next experiments were designed to determine whether the activation of pFRG during hypercapnia depends on glutamatergic synapses. Bilateral Kyn injections were then performed in the pFRG (100 mM), and the effects on hypercapnia-induced changes in PN, AbN, and tSN activities of in situ preparations were evaluated (Fig. 7). With respect to baseline parameters, the antagonism of glutamatergic receptors in the pFRG with Kyn microinjections did not alter resting perfusion pressure (74 ± 4 vs. before injections: 77 ± 4 mmHg), PN burst frequency (27 ± 4 vs. before injections: 21 ± 2 burst/min), and amplitude (12.4 ± 5.2 vs. before injections: 12.6 ± 4.4 µV), AbN mean activity (5.8 ± 0.5 vs. before injections: 6.1 ± 0.7 µV), and tSN inspiratory/postinspiratory activity (17.3 ± 4.3 vs. before injections: 15.2 ± 3.8 µV) of in situ preparations (n = 7).
Fig. 7.
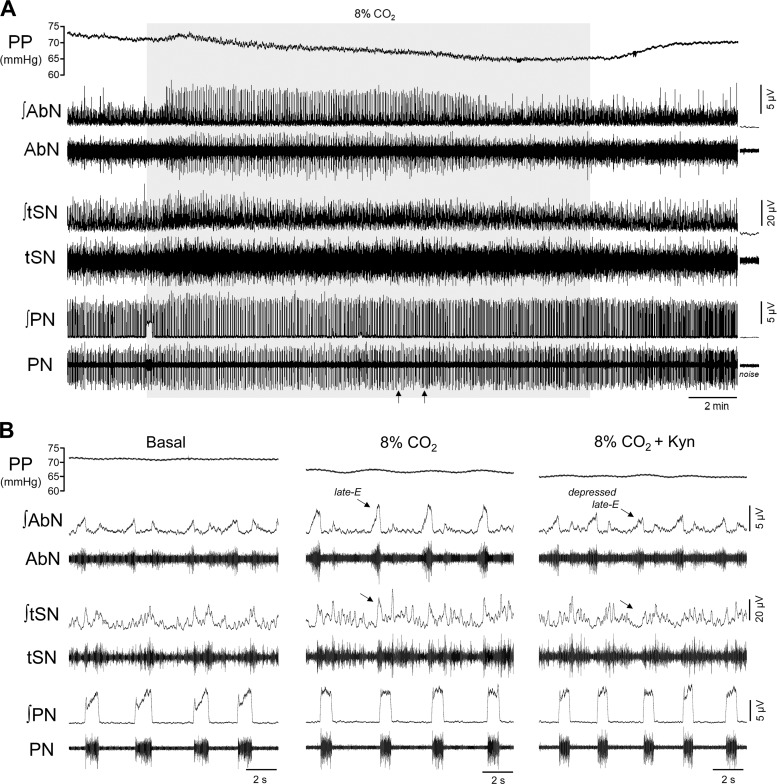
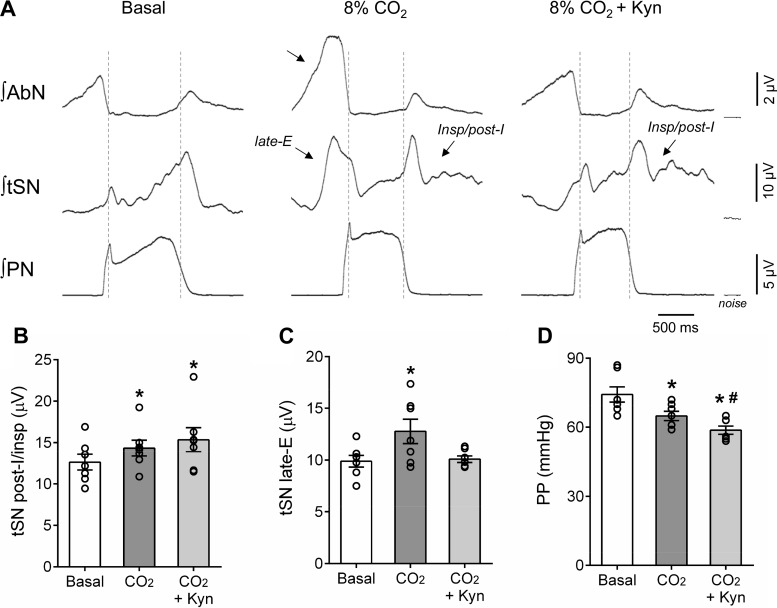
Respiratory and sympathetic reflex responses to hypercapnia after the antagonism of glutamatergic receptors in the parafacial respiratory group (pFRG). A: recordings of perfusion pressure (PP) and raw and integrated (∫) activities of the abdominal nerve (AbN), thoracic sympathetic verve (tSN), and phrenic nerve (PN) from a representative preparation, illustrating the effects of bilateral kynurenic acid (Kyn) injections in the pFRG region (arrows) on the respiratory and sympathetic reflex responses to hypercapnia (8% CO2, gray box). B: expanded recordings from A, demonstrating the level of PP and the pattern of AbN, tSN, and PN during eucapnia (basal), hypercapnia (8% CO2), and after the injections of Kyn in the pFRG (8% CO2 + Kyn). Note the emergence of late-expiration bursts in AbN and tSN during hypercapnia (arrows), which were markedly attenuated after the antagonism of glutamatergic receptors in the pFRG.
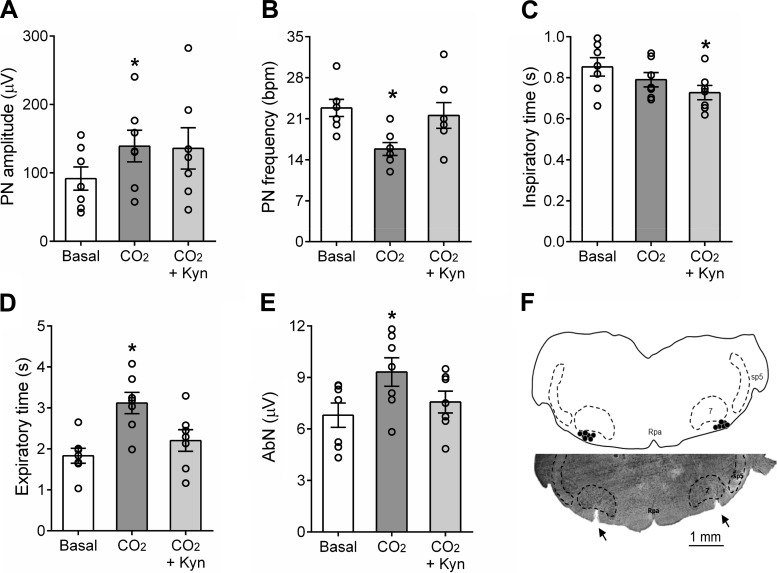
The raise of the CO2 fractional concentration in the perfusate to 8% increased the PN burst amplitude (139 ± 23 vs. baseline 92 ± 17 µV; ∆ = 43 ± 11%; P = 0.0169; Figs. 7, A and B and 8A), reduced the PN burst frequency (16 ± 1 vs. baseline: 23 ± 1 burst/min, P = 0.0041; Figs. 7B and 8B), did not produce significant changes in the inspiratory time (0.790 ± 0.034 vs. baseline: 0.852 ± 0.044 s; Fig. 8C), prolonged the expiratory time (3.121 ± 0.260 vs. baseline: 1.832 ± 0.183 s; P = 0.0078, Fig. 8D), and evoked late-E bursts in the AbN activity (6.8 ± 0.7 vs. baseline 9.3 ± 0.8; ∆ = 29 ± 7%; P = 0.0298; Figs. 7, A and B and 8E). Hypercapnia also increased the levels of SN activity during inspiration/postinspiration (14.3 ± 0.9 vs. baseline 12.6 ± 0.9; Δ = 17 ± 2%, P = 0.0042; Figs. 7B and 9, A and B) and generated novel bursts in the tSN during late-E phase (9.9 ± 0.6 vs. baseline 12.8 ± 1.2; ∆ = 39 ± 10%, P = 0.0412; Figs. 7B and 9, A–C). During hypercapnia, a significant decrease in the perfusion pressure was also noted (65 ± 2 vs. baseline: 74 ± 3mmHg; P = 0.0029, Figs. 7, A and B and 9D).
Fig. 8.
Effects of glutamatergic receptor antagonism in the parafacial respiratory group (pFRG) on the respiratory responses to hypercapnia. Average values of phrenic nerve (PN) burst amplitude (A) and frequency (B), times of inspiration (C) and expiration (D), and abdominal nerve (AbN) (E) late-expiration activity of in situ rat preparations (n = 7) during eucapnia (basal), hypercapnia (8% CO2), and after the injections of Kyn in the retrotrapezoid nucleus (RTN) (8% CO2 + Kyn). *Different from basal values, P < 0.05. F: schematic drawing combined with a photomicrography of a coronal section from a representative in situ preparation, illustrating the sites of injections (black dots) and pipette tracks (arrows) in the pFRG region. 7, facial nucleus; Rpa, raphe pallidus; sp5, spinal trigeminal tract.
Fig. 9.
Effects of glutamatergic receptor antagonism in the parafacial respiratory group (pFRG) on the pattern of sympathetic activity during hypercapnia. A: cycle-triggered averages of integrated (∫) abdominal nerve (AbN), thoracic sympathetic nerve (tSN), and phrenic nerve (PN) activities of a representative preparation, showing the tSN burst pattern during eucapnia (basal, A), hypercapnia (8% CO2), and after injections of kynurenic acid (Kyn) in the pFRG (8% CO2 + Kyn) of in situ preparations (n = 7). Note that hypercapnia amplified tSN during inspiratory/postinspiratory periods and introduced a novel burst during late-expiration (late-E) phase (arrows). The latter was associated with the emergence of late-E bursts in abdominal activity (AbN). B–D: average values of tSN during inspiratory/postinspiratory and late-E phases and of perfusion pressure (PP) respectively. *Different from basal, P < 0.05; #different from CO2, P < 0.05.
Bilateral injections of Kyn in the pFRG region during hypercapnia (Fig. 7A) slightly attenuated the increase in the PN burst amplitude (∆ = 38 ± 17%, Figs. 7, A and B and 8A), restored the PN burst frequency (22 ± 2 burst/min; Figs. 7B and 8B), decreased the inspiratory time (0.728 ± 0.035 s; P = 0.0228; Fig. 8C) and normalized the expiratory time (2.205 ± 0.264 s; Fig. 8D). Remarkably, the antagonism of glutamate receptor in the pFRG region markedly reduced the late-E bursts in AbN (∆ = 4 ± 6%; Figs. 7, A and B and 8E) and tSN (∆ = 14 ± 11%; Figs. 7A and 9, A and C). No changes were observed in the augmented tSN activity during inspiratory/postinspiratory period (∆ = 22 ± 7%; P = 0.0489; Fig. 9, A and B). Kyn injections in the pFRG region produced a further reduction in the perfusion pressure (Δ = 59 ± 2 mmHg; P = 0.0405; Figs. 7, A and B and 9D). All injection sites in the pFRG region were located ventral to the caudal pole of the facial nucleus (Fig. 8F), approximately between −11.52 and −11.78 mm relative to bregma (40). Complete recovery from the effects of bilateral injections of Kyn occurred within 60 min (responses in relation to baseline; ∆PN freq: −6 ± 2 burst/min; ∆PN amp: 26 ± 8%; ∆AbN late-E: 27 ± 5%; ∆tSN insp/post-I: 20 ± 4%; ∆tSN late-E: 20 ± 9%, P < 0.05).
The pFRG receives inputs from the RTN phox2b-positive neurons.
Three cases with a CTb injection in the pFRG were analyzed to visualize retrograde labeling in the RTN region (Fig. 10A). The distribution of representative CTb injections centered on the pFRG of three rats is shown in Fig. 10E. Phox2b-ir was used to identify the chemosensitive RTN neurons (Fig. 10C). RTN phox2b-expressing neurons with projections to the pFRG were labeled with CTb 7 to 10 days before the analysis. To characterize the RTN neurons that project to pFRG, a series of 40-μm thick coronal slices were analyzed, and cells were counted in 7 levels of RTN region (from −10.16 to −11.60 mm relative to Bregma) to include the lowest possible amount of catecholaminergic neurons in the RVLM/C1 neurons. A considerable number of retrogradely labeled neurons (CTb-ir) was found in the RTN region (Fig. 10, A and F), particularly in the VMS. Interestingly, as illustrated in Fig. 10, D and F, within the RTN region, the majority of retrogradely CTb-labeled neurons were immunoreactive for phox2b. We found from a total of 89 ± 41 CTb-ir neurons, 83 ± 38 phox2b-ir neurons that project to pFRG region (Phox2b-ir/CTb-ir), representing 93% (Fig. 10F), suggesting that chemically coded RTN cells project laterally to the pFRG.
Modeling and simulations.
The SSP-SAP and Kyn experiments were implemented in the framework of our computational model to provide a theoretical explanation of the excitatory mechanism required for the emergence of active expiration. Previously, we described central chemoreception as a tonic CO2-sensitive drive originating from the RTN (26, 29), and the emergence of active expiration during hypercapnia was attributed to an increase in the magnitude of this drive to the late-E population in the pFRG (Fig. 11). In the model, the late-E (pFRG) population is under phase-spanning inhibitory control from the rCPG (26, 43). During hypercapnia, an increase in CO2-sensitive RTN drive allows the late-E (pFRG) population to reach activation threshold at the end of expiration, at which time inhibition from the decrementing post-I (BötC) population becomes weak enough for the late-E population to activate. These late-E busts are visible in both the AbN, whose pattern in the model is formed solely by a projection from late-E (pFRG), and the tSN, whose pattern is formed by the RVLM.
Fig. 11.
Model connectivity diagram and manipulations. Our model of the respiratory and sympathetic circuitry includes structures in the pons, the ventral respiratory column (VRC), the retrotrapezoid nucleus (RTN), and the parafacial respiratory group (pFRG). Orange circles and blue circles, respectively, represent excitatory and inhibitory neuronal populations. Solid green triangles represent sources of tonic drive. Orange and blue solid arrows, respectively, represent excitatory and inhibitory synaptic projections; solid green arrows indicate the distribution of tonic drive. The details of tonic drive distribution [e.g., from drive RTN, drive Pons, and drive ventrolateral medulla (VLM)] can be found in Table 1. Brown circles indicate motoneuron output. The included neural populations are the early inspiratory [early-I (1)] and preinspiratory/inspiratory (pre-I/I) populations of the pre-Bötzinger complex (pre-BötC), the inhibitory postinspiratory (post-I), excitatory postinspiratory [post-I (e)], and the augmenting expiratory (aug-E) populations of the BötC, the ramping inspiratory (ramp-I) and early inspiratory [early-I (2)] populations of the rostral ventral respiratory group (rVRG), the inspiratory-expiratory phase spanning (IE) population of the pons, the late expiratory (late-E) population of the pFRG, and the presympathetic population of the rostral VLM (RVLM). We have implemented experimental manipulations in the model by updating certain model parameters. For example, the galf-filled green triangle indicates a source of tonic drive that is impaired to reproduce saporin-substance P conjugate (SSP-SAP) lesion experiments. Projections that have been removed to simulate glutamatergic blockade are indicated as a dashed line. AbN, abdominal nerve activity; CPG, central pattern generator; cVN, central vagus nerve activity; HN, hypoglossal nerve activity; Kyn, kynurenic acid; PN, phrenic nerve activity; tSN, thoracic sympathetic nerve activity.
We attributed the suppression of late-E activity during hypercapnia observed in the SSP-SAP experiments and the Kyn experiments to a perturbation of the RTN drive, and such perturbations are formulated in the context of our computational model of the rCPG and the expiratory oscillator. The SSP-SAP manipulation was implemented in the model as an overall decrease in the CO2-sensitive RTN drive by 50% (see METHODS) (Fig. 11). In the rCPG, this change affected both the inspiratory pre-I/I (pre-BötC) and early-I (pre-BötC) populations as well as the expiratory aug-E (BötC) population in a relatively balanced fashion. The Kyn manipulation was implemented in the model by eliminating the projection from RTN to late-E (pFRG) (Fig. 11).
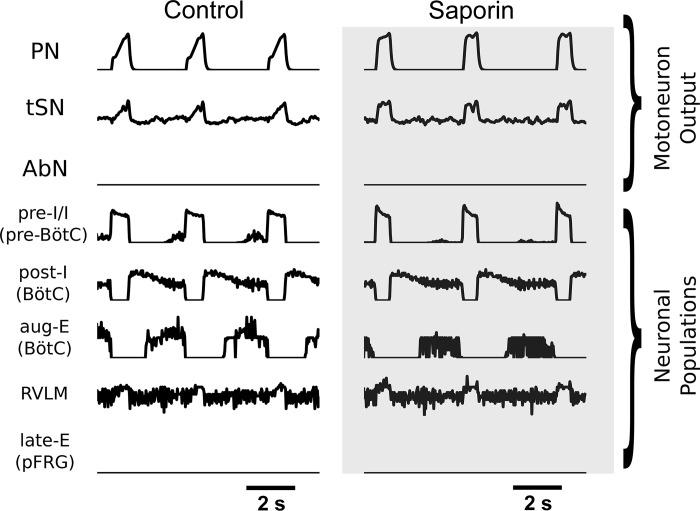
Decrease in balanced excitatory input from the RTN to the rCPG decreases baseline inspiratory duration and amplitude in motoneuron output.
Our model reproduced the change to inspiratory parameters of motoneuron output following RTN neuron lesions with SSP-SAP (Fig. 12). In the present model, the RTN lesion manipulation was designed to reduce drive to both expiratory and inspiratory populations in a balanced fashion: reduced drive to aug-E (BötC) prevents expiration time from increasing substantially and reduced drive to early-I/I (1) (pre-BötC) and pre-I/I (pre-BötC) allows the inspiratory duration to shrink. In this way, the strong reduction of RTN input to the CPG in the SSP-SAP simulation does not abolish the three-phase rhythm. The results of these manipulations are visible in the CPG activity and motoneuron output; both inspiratory activity and aug-E bursts are diminished (Fig. 12). The duration and amplitude of the PN burst is reduced, and the amplitude of inspiratory component of tSN activity is reduced (Fig. 12).
Fig. 12.
Simulations of eucapnia control and saporin-substance P conjugate (SSP-SAP) conditions. Motoneuron output for the phrenic nerve (PN), the thoracic sympathetic nerve (tSN), and the abdominal nerve (AbN) are presented for the control simulation and the simulation of animals with SSP-SAP retrotrapezoid nucleus (RTN) lesions. Select neuronal populations included are the preinspiratory/inspiratory (pre-I/I) population of the pre-Bötzinger complex (pre-BötC), the postinspiratory and augmenting expiratory populations of the BötC, the presympathetic population of the rostral ventrolateral medulla (RVLM), and the late-expiratory (late-E) population of the parafacial respiratory group (pFRG).
Active expiration is suppressed by reduction or removal of the RTN drive to the pFRG.
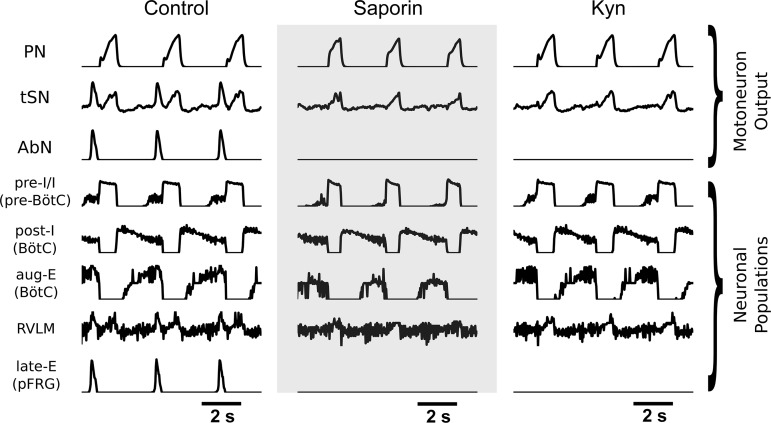
The model reproduced the suppression of active expiration observed during hypercapnia in the SSP-SAP experiments. In all simulations, the CO2-sensitive RTN drive was increased from the respective baseline to implement hypercapnia (see Methods for details) and induce the emergence of late-E activity characteristic to active expiration (Fig. 13). These late-E bursts are visible in both the AbN and tSN outputs. In the control simulation of hypercapnia, an increase in drive from the RTN allows the late-E (pFRG) population to reach threshold at the end of expiration, as inhibition from post-I (BötC) is diminished over time. However, in simulations of the SSP-SAP experiments performed in hypercapnia, where the RTN drive is substantially weakened, the CO2 drive is not great enough during hypercapnia for the late-E (pFRG) population to reach activation threshold. Also, with the reduction of RTN drive, the inspiratory and sympathetic responses diminish (Fig. 13).
Fig. 13.
Simulations of hypercapnia control, saporin-substance P conjugate (SSP-SAP) and kynurenic acid (Kyn) conditions. Motoneuron output for the phrenic nerve (PN), the thoracic sympathetic nerve (tSN), and the abdominal nerve (AbN) are presented for the control simulation, the simulation of animals with SSP-SAP retrotrapezoid nucleus (RTN) lesions, and the simulation of parafacial respiratory group (pFRG) Kyn injections. Select neuronal populations included are the preinspiratory/inspiratory (pre-I/I) population of the pre-Bötzinger complex (pre-BötC), the postinspiratory and augmenting expiratory populations of the BötC, the presympathetic population of the rostral ventrolateral medulla (RVLM), and the late-expiratory (late-E) population of the parafacial respiratory group (pFRG).
The model also reproduced the suppression of active expiration following the antagonism of glutamatergic neurotransmission with Kyn in the pFRG. In this simulation, there is no manipulation to the RTN integrity or sensitivity of the RTN to hypercapnia, but the projection from the RTN to the late-E (pFRG) population is removed (Fig. 11). Here, the pFRG population does not receive RTN drive during hypercapnia, and there is no mechanism for it to overcome expiratory inhibition from the rCPG (Fig. 13). Consequently, no late-E activity is observed in the AbN and tSN outputs. In contrast, the increase in PN amplitude and in the inspiratory-related component of tSN is preserved (Fig. 13).
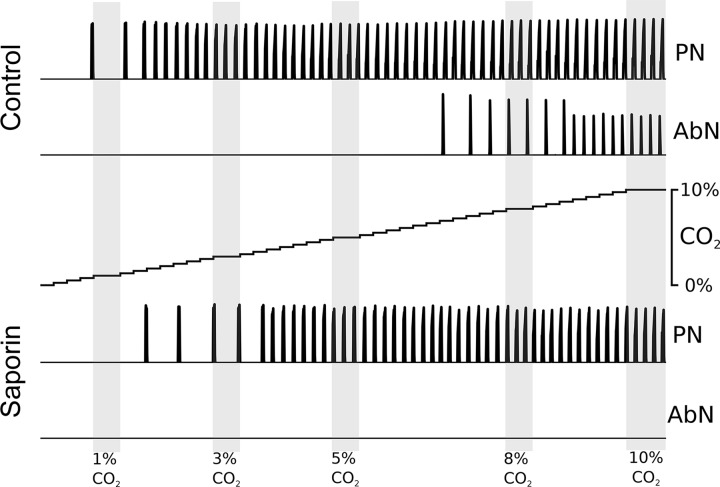
Apneic threshold is increased by reduction of the RTN drive.
Previous studies reported an increase in the apneic threshold in animals subjected to lesions of NK1R-expressing neurons in the RTN (51). This model also reproduces these previous results (Fig. 14). We performed simulations in which the CO2 percentage was a linear function of time that swept the parameter space from extreme hypocapnia through eucapnia into hypercapnia. In the control model, inspiratory bursts were observed at the RTN drive value that corresponded to 1% CO2, and regular respiratory activity was observed beginning at ~2% CO2. In this model, late-E abdominal bursts appeared at ~7% CO2. In the SSP-SAP model, the RTN drive became sufficient to initiate inspiratory bursts at ~2% CO2, but regular respiratory activity did not appear until ~4% CO2. Active expiration did not appear in the SSP-SAP simulations, even at 10% CO2.
Fig. 14.
In simulation, the control model is more sensitive to CO2 concentration. Motoneuron output for the phrenic nerve (PN) and the abdominal nerve (AbN) are depicted for simulations with gradually increasing CO2 partial pressure for the control model and the saporin-substance P conjugate (SSP-SAP) lesion model. CO2 partial pressure is incremented in steps of 0.25% from 0% to 10%. Lightly shaded regions emphasize activity at 1%, 3%, 5%, 8%, and 10% CO2.
DISCUSSION
In conditions of elevated levels of inspired CO2, chemosensitive cells in the central nervous system are stimulated and provide excitatory drive to rCPG and sympathetic nervous system to generate respiratory and autonomic responses. Recent studies identified that during hypercapnia, expiration transforms into an active process, with the generation of late-E bursts in the abdominal motor activity (1, 29). The emergence of active expiration during hypercapnia has been suggested as an important step to increase pulmonary ventilation (28), as well as to introduce novel respiratory-related discharge bursts in the sympathetic activity (29). In the present study, we present new physiological and neuroanatomical evidence that supports the notion that the phox2b-expressing neurons of the RTN region interact with the functionally distinct neurons of the pFRG to provide the excitatory drive necessary for the generation of active expiration and sympathoexcitation in rats.
SSP-SAP toxin destroys RTN chemosensitive cells and reduces baseline respiratory and sympathetic activities.
Previous studies demonstrated that the SSP-SAP selectively destroys neurons that express NK1R and spares neurons that express other types of substance P receptors (12, 37, 48, 51, 54). Although very little NK1R-immunoreactivity associated with neuronal cell bodies is visible in the RTN, some form of neurokinin receptor is present on the surface of the phox2b-positive neurons because these cells are strongly activated by substance P both in vitro and in vivo (35, 48). At the dose used in the present study, we found that the SSP-SAP microinjections eliminated ~85% of NK1R-expressing neurons and ~63% of phox2b-positive neurons in the RTN. Importantly, our SSP-SAP microinjections did not cause lesions of cholinergic neurons in the facial motor nucleus, nucleus ambiguus, dorsal motor nucleus of the vagus, and hypoglossal motor nucleus. Reductions in the NK1R immunoreactivity were also noted in the pyramidal tract, which contains neurons that extend their dendrites into the RTN region. However, studies demonstrated that these neurons do not play a significant role in the responses to hypercapnia (36). SSP-SAP microinjections in the RTN also reduced the expression of NK1R in the BötC/RVLM region. Previous studies reported that C1 neurons of RVLM express NK1Rs and are responsive to substance P (21). There is also evidence suggesting that part of expiratory neurons of the BötC may express NK1R (11). Importantly, we verified that our SSP-SAP microinjections in the RTN did not cause significant reductions of C1 neurons (phox2b+/TH+). Based upon that, we speculate that SSP-SAP microinjections in the RTN caused lesions of some non-C1 and expiratory neurons in the RVLM/BötC region, which cannot be ruled out as an additional mechanism contributing to the changes in respiratory and sympathetic activities in SSP-SAP-lesioned rats. However, different from our experiments, nonselective inhibition of the BötC/RVLM region markedly depresses the respiratory rhythm and attenuates the respiratory and sympathetic responses to a peripheral chemoreceptor (23, 32). Therefore, despite of the reduction of NK1R-ir in the BötC/RVLM region, we consider that the observed changes in respiratory and sympathetic activities in SSP-SAP lesioned rats were mainly dependent on reductions of phox2b-expressing neurons in the RTN region.
The in situ preparations of rats with lesions of NK1R-expressing neurons in the RTN exhibited reduced phrenic burst duration at resting eucapnic conditions. In adult unrestrained awake rats, bilateral injections of SSP-SAP in the RTN diminished baseline tidal volume (37, 48). Therefore, we suggest that the shorter inspiratory time may be a mechanism contributing to reduced tidal volume observed in vivo after SSP-SAP injections in the RTN. We proposed that these changes in resting inspiratory activities produced by bilateral injections of SSP-SAP into the RTN are likely due to the loss of the phox2b-expressing neurons in this area, with consequent reductions of CO2-dependent excitatory drive to rCPG (24, 29). In association with the reduced inspiratory activity, we identified that in situ preparations of SSP-SAP-lesioned rats exhibited lower baseline sympathetic activity and weaker respiratory-related bursts. There is evidence indicating that baseline respiratory modulation of sympathetic activity is mainly determined by respiratory inputs to C1 neurons of RVLM (25, 33). By the fact that our SSP-SAP microinjections in the RTN did not cause lesions of C1 neurons in the RVLM, we suggested that the reduced CO2 drive from RTN to rCPG also contributes to depress the respiratory-related bursts in sympathetic activity. These observations agree with our previous findings showing that acute pharmacological inhibition of the RTN (with injections of GABAA receptor agonist) reduced phrenic and sympathetic activities (32, 50).
Retrotrapezoid chemosensitive neurons drive active expiration and expiratory-sympathetic coupling during hypercapnia.
Accumulating evidence supports the notion that the RTN neurons are intrinsically sensitive to CO2/H+ (36, 55) and necessary for the central chemoreception (19, 47, 50). In adult rats, bilateral injections of SSP-SAP in the RTN resulted in a significant reduction of the number of phox2b neurons, accompanied by a large reduction of the sympathetic and ventilatory responses to hypercapnia (37, 48, 51). Herein, we found that SSP-SAP microinjections in juvenile rats elicited a significant reduction of phox2b+ neurons in the RTN region (~63%) and greatly attenuated the increase in phrenic burst amplitude elicited by high CO2 in the in situ preparations, confirming previous observations. Additionally, we found that the expiratory response to hypercapnia was markedly reduced after lesions of NK1R-positive neurons of the RTN. Considering that the recruitment of expiratory pumping muscles is an important mechanism to enhance pulmonary ventilation, mainly contributing to increased tidal volume (17, 20), our data indicate that the reduced ventilatory response to hypercapnia in SSP-SAP-lesioned rats results from attenuation of both inspiratory and expiratory motor activities. We also verified that in the absence of anesthesia, the sympatho-excitatory response to hypercapnia was attenuated in SSP-SAP-lesioned rats. This reduction was associated with significant reductions of the inspiratory- and expiratory-related components of sympathetic activity during hypercapnia. Taken together, our data further support that chemosensitive neurons of the RTN play a critical role in processing the inspiratory, expiratory, and sympathetic adjustments under hypercapnia.
There is evidence showing that part of the pFRG neurons also express NK1R (14). Studies performing viral injections in the pFRG region to express the Gq-coupled HM3D DREADD receptor in the NK1R-expressing neurons suggest that the stimulation of NK1R-positive neurons of the pFRG is able to increase both diaphragm and abdominal expiratory activities (15). Although this previous study has not functionally characterized the pFRG neurons that express NK1R (chemosensitive vs. expiratory), one might speculate that the elimination of active expiration elicited by hypercapnia in SSP-SAP-lesioned rats was consequent to lesions in some of the pFRG expiratory neurons. However, we found that stimulation of peripheral chemoreceptors in SSP-SAP-lesioned rats was able to rescue abdominal late-E activity, which was significantly reduced during hypercapnia. These findings indicate that the expiratory neurons of the pFRG were not lesioned by the microinjections of SSP-SAP, and their activation during hypercapnia was markedly attenuated by the loss of NK1R-expressing chemosensitive neurons in the RTN region.
Glutamatergic neurotransmission within the pFRG region is necessary for the emergence of active expiration and sympathetic overactivity during hypercapnia.
Previous studies by Huckstepp and colleagues (14), using ubiquitous viral transfection approaches, demonstrated that activation of neurons located ventromedial to the caudal pole of the facial nucleus, corresponding to the region where the RTN phox2b+ neurons reside, stimulates both inspiratory and expiratory motor activities, whereas activation of neurons located lateral to the caudal part of facial nucleus, corresponding to the pFRG, increases mostly the abdominal expiratory activity. In our study, we found that the antagonism of glutamatergic receptors in the pFRG during hypercapnia markedly attenuated the late-E bursts in the AbN and tSN activities but did not modify the PN response as well as the inspiratory-related bursts in the tSN activity. This pattern of response differs from the responses obtained in rats that received SSP-SAP injections in the RTN, in which all respiratory and sympathetic responses to hypercapnia were attenuated. These findings strongly support the notion that the RTN and the pFRG are two functionally distinct populations of neurons, i.e., one responsible for the detection of CO2/H+ levels, presumably through intrinsic chemosensitive properties that are not affected by Kyn injections, and another responsible for the generation of active expiration, which requires glutamatergic neurotransmission to be recruited during hypercapnia. Interestingly, our anatomical data demonstrate the existence of projections from phox2b neurons to the pFRG region. We cannot rule out the possibility that some retrogradely labeled phox2b neurons might have picked up the dye by distal dendrites that reached the injection area. However, these anatomical data, in conjunction with our functional, indicate for the first time that the RTN and pFRG neurons form a microcircuitry where the RTN phox2b-positive neurons send glutamatergic inputs to the pFRG and thus stimulate the late-E neurons under high levels of CO2. These novel data help us advance our understating of the neurochemical mechanisms required to recruit the expiratory oscillator and generate the pattern of active expiration under physiological stimulation.
In addition to active expiration, the glutamatergic neurotransmission in the pFRG region also contributes to the sympathoexcitatory response to high CO2. It is suggested that excitatory inputs from the RTN neurons to the presympathetic neurons of the RVLM are a major drive to increase sympathetic outflow during hypercapnia (34, 49). However, our data showing that microinjections of Kyn in the pFRG attenuated the late-E bursts in tSN during high CO2, but not the amplification in the inspiratory-related burst, suggest that both RTN and pFRG neurons may interact with RVLM neurons. In this scenario, we propose that excitatory drive from the chemosensitive population may provide a tonic excitatory component to the RVLM and contribute to amplify the overall sympathetic activity, including the inspiratory/postinspiratory component, whereas excitatory inputs from the pFRG, driven by the RTN, are determinant to increase RVLM and sympathetic activities specifically during late-E phase. Although additional experiments are still required to confirm anatomical connections between the pFRG and the RVLM, previous studies have evidenced an association between the recruitment of expiratory neurons of the pFRG and the emergence of increased discharge frequency of the RVLM presympathetic neurons during late expiratory phase (30, 31), supporting our hypothesis. Importantly, by the fact that the elimination of late-E bursts in tSN was accompanied by a reduction in the perfusion pressure of in situ preparations, we propose that this expiratory-related burst is functionally relevant and contributes to increased vascular resistance during hypercapnia.
The theoretical model predicts the interactions among RTN/pFRG, rCGP, and RVLM during hypercapnia.
In simulation, we demonstrated that an overall reduction in the output and sensitivity to CO2 of the RTN was sufficient to reproduce the absence of active expiration in lesion experiments. The experimental condition of lesioned NK1R-expressing chemosensitive neurons in the RTN was simulated in our model by reducing CO2-dependent drive to other compartments by 50%. This value best reproduced our experimental data obtained in situ as well as the increase in apneic threshold reported in vivo (51). In our experimental conditions, the NK1R immunoreactivity in SSP-SAP treated rats was reduced, on average, to 15% that of control animals (Fig. 1). The apneic threshold in situ is generally found between 1% and 3% perfusate CO2 (29), which is ~40% of the eucapnic level. However, we found that SSP-SAP-lesioned rats did not experience respiratory failure at eucapnia despite the significantly reduced input from the RTN. In the model, reductions in the RTN drive to levels comparable to the change in immunoreactivity would substantially impair the respiratory rhythm. Therefore, we speculate that there may be other sources of excitatory drive in the RTN to the respiratory CPG that do not express NK1R and therefore are not prone to SSP-SAP.
As a consequence of the reduction in the RTN drive to all compartments (SSP-SAP simulations), 1) the inspiratory motor output as well as the inspiratory-related burst amplitude in the sympathetic output decreased, and 2) active expiration and the late-E sympathetic activity under hypercapnia were suppressed. Thus, the proposed connections in the model successfully accommodated the experimental observations obtained from Kyn injections in the pFRG, by reducing the excitatory RTN input to the pFRG. With this, baseline and hypercapnia-induced inspiratory-related activity in the phrenic and sympathetic outputs were preserved, whereas the late-E activity in the abdominal and sympathetic output was abolished. The model, therefore, qualitatively and quantitatively reproduces all experimental findings of the present study, thus substantiating the underlying assumptions about RTN and pFRG circuit organization and their role in the formation of abdominal and sympathetic motor output during hypercapnia.
In our computational model, the RTN sends excitatory input to inspiratory [early-I (pre-BötC) and pre-I/I (pre-BötC)] and expiratory populations [aug-E (BötC) and late-E (pFRG)] to provide the CO2-dependent drive to breath, as seen in vivo. In agreement with previous observations (2, 29), the respiratory-sympathetic coupling is suggested to be generated through connections between the sympathetic RVLM population with the VRC neurons and the phase-spanning pontine neurons. With the elevation of the RTN drive (to mimic hypercapnia condition), the respiratory and sympathetic activities are enhanced due to excitation of neuronal populations in the BötC, pre-BötC, and RVLM. With respect to the late-E component, the RTN-pFRG interaction (verified experimentally in our present study) was suggested to provide the necessary excitatory drive to the late-E (pFRG) population that overcame the inhibition to the pFRG [presumably from the post-I (BötC) and early-I (pre-BötC) populations] and generate the late-E discharges in the abdominal activity. The emergence of the late-E (pFRG) population also brought about late-E bursts in sympathetic activity through interactions with the RVLM population. In the model, we proposed a direct excitatory projection from the pFRG to the RVLM. However, we cannot exclude the possibility of indirect pathways, including through the neurons of the caudal ventrolateral medulla (22).
In aggregate, the perturbations of the input to the respiratory CPG, the conditional expiratory oscillatory, and presympathetic circuitry serve to further validate the computational framework that integrates existing data on the physiology of brainstem respiratory circuitry and its interactions with essential homeostatic responses.
Summary and conclusions.
In conclusion, our study supports the view that in the ventral surface of medulla, rostral to the VRC, two groups of functionally distinct neurons reside ventral to the facial nucleus (14). One of these populations is the classical chemosensitive phox2b-positive neurons of the RTN that intrinsically detect the levels of CO2/H+ and provide excitatory drive to respiratory and sympathetic neurons in the brainstem (13). The other is the expiratory population of the pFRG, which comprises the conditional expiratory oscillator (16). Our study provides, for the first time evidence that these two populations interact in a way that glutamatergic inputs from the RTN to the pFRG are necessary for the activation of the expiratory neurons during hypercapnia, which is responsible for the generation of active expiratory patterns and the emergence of expiratory-related bursts in sympathetic activity. The revealed RTN and pFRG microcircuitry helps us understand the neural organization of the rCPG and the control of respiratory and sympathetic responses in conditions of increased arterial CO2 content. Thereby, our findings have potential implications for understanding the development of cardiorespiratory dysfunctions, associated blood gas disturbances, and activation of chemoreceptors, as observed in patients with heart failure, obstructive and central apneas, chronic obstructive pulmonary disease, and others.
GRANTS
This work was supported by São Paulo Research Foundation Grants 2013/17251-6 (to D. B. Zoccal), 2009/54888-7 (to E. Colombari), 2015/23376-1 and 2016/22069-0 (to T. S. Moreira), and 2014/22406-1 and 2016/23281-3 (to A. C. Takakura); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grants 471283/2012-6 (to T. S. Moreira) and 471263/2013-3 (to A. C. Takakura); National Institutes of Health Grants R01-AT-008632 and U01-EB-021960 (to Y. I. Molkov); and CNPq fellowships 302892/2014-1 (to D. B. Zoccal), 301904/2015-4 (to T. S. Moreira), and 301219/2016-8 (to A. C. Takakura).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.B.Z., W.H.B., E.C., Y.I.M., T.S.M., and A.C.T. conceived and designed research; D.B.Z., J.N.S., W.H.B., E.V.L., B.F., E.C., Y.I.M., T.S.M., and A.C.T. performed experiments; D.B.Z., J.N.S., W.H.B., E.V.L., B.F., Y.I.M., T.S.M., and A.C.T. analyzed data; D.B.Z., J.N.S., W.H.B., E.V.L., B.F., E.C., Y.I.M., T.S.M., and A.C.T. interpreted results of experiments; D.B.Z., J.N.S., W.H.B., E.V.L., B.F., Y.I.M., T.S.M., and A.C.T. prepared figures; D.B.Z., T.S.M., and A.C.T. drafted manuscript; D.B.Z., J.N.S., W.H.B., E.V.L., B.F., E.C., Y.I.M., T.S.M., and A.C.T. edited and revised manuscript; D.B.Z., J.N.S., W.H.B., E.V.L., B.F., E.C., Y.I.M., T.S.M., and A.C.T. approved final version of manuscript.
REFERENCES
- 1.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539–3559, 2009. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008. doi: 10.1113/expphysiol.2007.041400. [DOI] [PubMed] [Google Scholar]
- 3.Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir Physiol Neurobiol 174: 135–145, 2010. doi: 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barna BF, Takakura AC, Moreira TS. Acute exercise-induced activation of Phox2b-expressing neurons of the retrotrapezoid nucleus in rats may involve the hypothalamus. Neuroscience 258: 355–363, 2014. doi: 10.1016/j.neuroscience.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Barna BF, Takakura AC, Moreira TS. Pontomedullary and hypothalamic distribution of Fos-like immunoreactive neurons after acute exercise in rats. Neuroscience 212: 120–130, 2012. doi: 10.1016/j.neuroscience.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Barnett WH, Abdala AP, Paton JF, Rybak IA, Zoccal DB, Molkov YI. Chemoreception and neuroplasticity in respiratory circuits. Exp Neurol 287: 153–164, 2017. doi: 10.1016/j.expneurol.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett WH, Jenkin SE, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB. The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J Neurophysiol 119: 401–412, 2018. doi: 10.1152/jn.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa-Silva JH, Zoccal DB, Machado BH. Glutamatergic antagonism in the NTS decreases post-inspiratory drive and changes phrenic and sympathetic coupling during chemoreflex activation. J Neurophysiol 103: 2095–2106, 2010. doi: 10.1152/jn.00802.2009. [DOI] [PubMed] [Google Scholar]
- 9.de Britto AA, Moraes DJ. Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats. J Physiol 595: 2043–2064, 2017. doi: 10.1113/JP273335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong AY, Potts JT. Neurokinin-1 receptor activation in the Bötzinger complex evokes bradypnea and is involved in mediating the Hering-Breuer reflex. Adv Exp Med Biol 605: 366–370, 2008. doi: 10.1007/978-0-387-73693-8_64. [DOI] [PubMed] [Google Scholar]
- 12.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci 35: 1052–1067, 2015. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huckstepp RT, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. eLife 5: e14203, 2016. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkin SE, Milsom WK. Expiration: breathing’s other face. Prog Brain Res 212: 131–147, 2014. doi: 10.1016/B978-0-444-63488-7.00008-2. [DOI] [PubMed] [Google Scholar]
- 18.Jenkin SE, Milsom WK, Zoccal DB. The Kölliker-Fuse nucleus acts as a timekeeper for late-expiratory abdominal activity. Neuroscience 348: 63–72, 2017. doi: 10.1016/j.neuroscience.2017.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA. Physiology. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348: 1255–1260, 2015. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemes EV, Zoccal DB. Vagal afferent control of abdominal expiratory activity in response to hypoxia and hypercapnia in rats. Respir Physiol Neurobiol 203: 90–97, 2014. doi: 10.1016/j.resp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Li YW, Guyenet PG. Effect of substance P on C1 and other bulbospinal cells of the RVLM in neonatal rats. Am J Physiol Regul Integr Comp Physiol 273: R805–R813, 1997. doi: 10.1152/ajpregu.1997.273.2.R805. [DOI] [PubMed] [Google Scholar]
- 22.Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 572: 881–896, 2006. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within pre-Bötzinger and Bötzinger complexes. eNeuro 3: 3, 2016. doi: 10.1523/ENEURO.0011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci 30: 12466–12473, 2010. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menuet C, Le S, Dempsey B, Connelly AA, Kamar JL, Jancovski N, Bassi JK, Walters K, Simms AE, Hammond A, Fong AY, Goodchild AK, McMullan S, Allen AM. Excessive respiratory modulation of blood pressure triggers hypertension. Cell Metab 25: 739–748, 2017. doi: 10.1016/j.cmet.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J Neurophysiol 104: 2713–2729, 2010. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molkov YI, Rubin JE, Rybak IA, Smith JC. Computational models of the neural control of breathing. Wiley Interdiscip Rev Syst Biol Med 9: e1371, 2017. doi: 10.1002/wsbm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molkov YI, Shevtsova NA, Park C, Ben-Tal A, Smith JC, Rubin JE, Rybak IA. A closed-loop model of the respiratory system: focus on hypercapnia and active expiration. PLoS One 9: e109894, 2014. doi: 10.1371/journal.pone.0109894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol 105: 3080–3091, 2011. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moraes DJ, Bonagamba LG, Costa KM, Costa-Silva JH, Zoccal DB, Machado BH. Short-term sustained hypoxia induces changes in the coupling of sympathetic and respiratory activities in rats. J Physiol 592: 2013–2033, 2014. doi: 10.1113/jphysiol.2013.262212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 33: 19223–19237, 2013. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of the retrotrapezoid nucleus/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J Neurophysiol 108: 882–890, 2012. doi: 10.1152/jn.00193.2012. [DOI] [PubMed] [Google Scholar]
- 33.Moraes DJ, Bonagamba LG, da Silva MP, Paton JF, Machado BH. Role of ventral medullary catecholaminergic neurons for respiratory modulation of sympathetic outflow in rats. Sci Rep 7: 16883, 2017. doi: 10.1038/s41598-017-17113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2006. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 37.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31: 2895–2905, 2011. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods 65: 63–68, 1996. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam, Boston: Academic Press/Elsevier, 2007. [Google Scholar]
- 41.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 29: 58–71, 2014. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24: 464–472, 2001. doi: 10.1016/S0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 43.Rubin JE, Bacak BJ, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Interacting oscillations in neural control of breathing: modeling and qualitative analysis. J Comput Neurosci 30: 607–632, 2011. doi: 10.1007/s10827-010-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva JN, Tanabe FM, Moreira TS, Takakura AC. Neuroanatomical and physiological evidence that the retrotrapezoid nucleus/parafacial region regulates expiration in adult rats. Respir Physiol Neurobiol 227: 9–22, 2016. doi: 10.1016/j.resp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takakura AC, Barna BF, Cruz JC, Colombari E, Moreira TS. Phox2b-expressing retrotrapezoid neurons and the integration of central and peripheral chemosensory control of breathing in conscious rats. Exp Physiol 99: 571–585, 2014. doi: 10.1113/expphysiol.2013.076752. [DOI] [PubMed] [Google Scholar]
- 49.Takakura AC, Colombari E, Menani JV, Moreira TS. Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol Regul Integr Comp Physiol 300: R501–R510, 2011. doi: 10.1152/ajpregu.00220.2010. [DOI] [PubMed] [Google Scholar]
- 50.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 586: 2975–2991, 2008. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci 11: 538–540, 2008. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taxini CL, Takakura AC, Gargaglioni LH, Moreira TS. Control of the central chemoreflex by A5 noradrenergic neurons in rats. Neuroscience 199: 177–186, 2011. doi: 10.1016/j.neuroscience.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 22: 3755–3764, 2002. doi: 10.1523/JNEUROSCI.22-09-03755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33: 7756–7761, 2013. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]