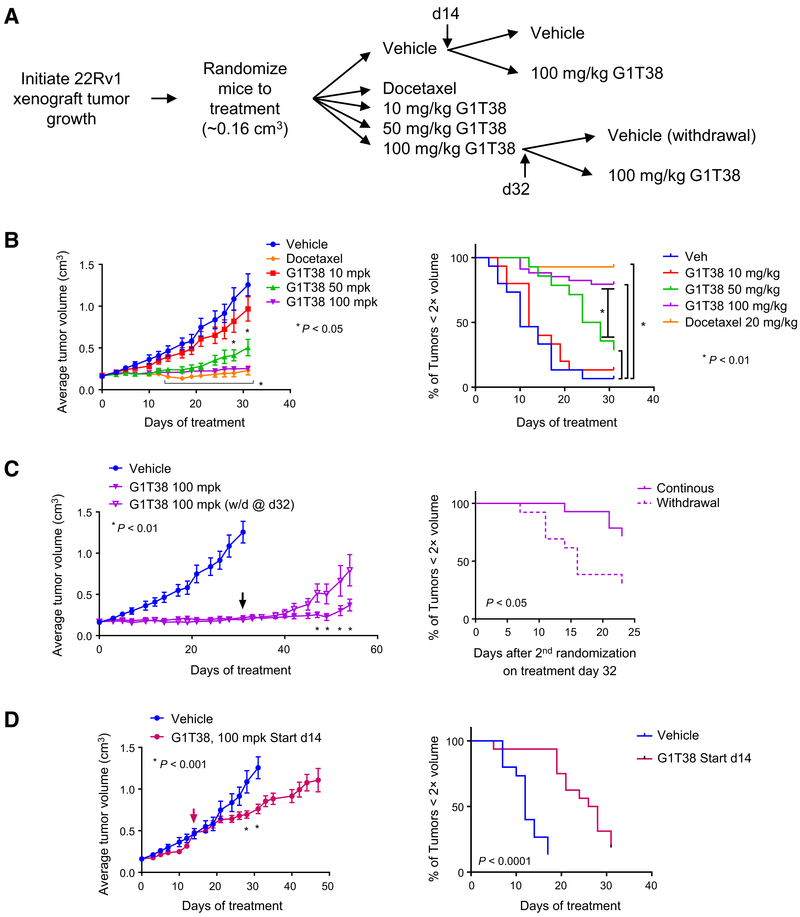
Figure 3.
Tumor growth suppression by CDK4/6i is dependent upon starting tumor volume and continuous exposure. A, Experimental outline: 22Rv1 xenograft tumors were initiated in castrated nu/nu mice and allowed to reach approximately 0.15 cm3 volume prior to randomization treatment with vehicle (n = 31), with 20 mg/kg docetaxel (intraperitoneally, every week), or with 10, 50, or 100 mg/kg G1T38 (orally, every day, n = 15, 14, and 27, respectively). On treatment day 14, vehicle-treated animals were rerandomized to continue to receive vehicle (n = 15) or to treatment with G1T38 (100 mg/kg orally, every day, n = 16). On treatment day 32, mice receiving 100 mg/kg G1T38 were rerandomized to continue to receive 100 mg/kg G1T38 (n = 14) or to treatment with vehicle (n = 13). Average tumor volume ± SEM of animals treated as described are presented in B–D, as well as time (days) required to reach 2× tumor volume as compared with initial volume on day 0. B, Average tumor volume ± SEM of all treatment groups during treatment days 0 to 31. Two-way ANOVA analysis followed by Bonferroni multiple comparison test determined that significant tumor growth inhibition (P < 0.05) was observed for 10 mg/kg G1T38 (days 28 and 31) and for 50 or 100 mg/kg G1T38 or docetaxel treatment (days 14–31). Standard Kaplan–Meier analysis of time to endpoint indicated a significant growth delay using an adjusted Bonferroni cutoff of P < 0.01. C, Averagetumor volume ± SEM of vehicle and 100 mg/kg G1T38 (continuous and withdrawal on treatment day 32). Two-way ANOVA analysis followed by Bonferroni multiple comparison test, as well as Kaplan–Meier analysis, determined that significant tumor growth (P < 0.05) was observed on treatment days 47 to 54 for animals rerandomized to vehicle after treatment day 31 (arrow). D, Average tumor volume ± SEM of vehicle and 100 mg/kg G1T38 (treatment days, 14–47). Two-way ANOVA analysis followed by Bonferroni multiple comparison test, as well as Kaplan–Meier analysis, determined that significant tumor growth inhibition (P < 0.05) was observed following rerandomization to treatment with 100 mg/kg G1T38.