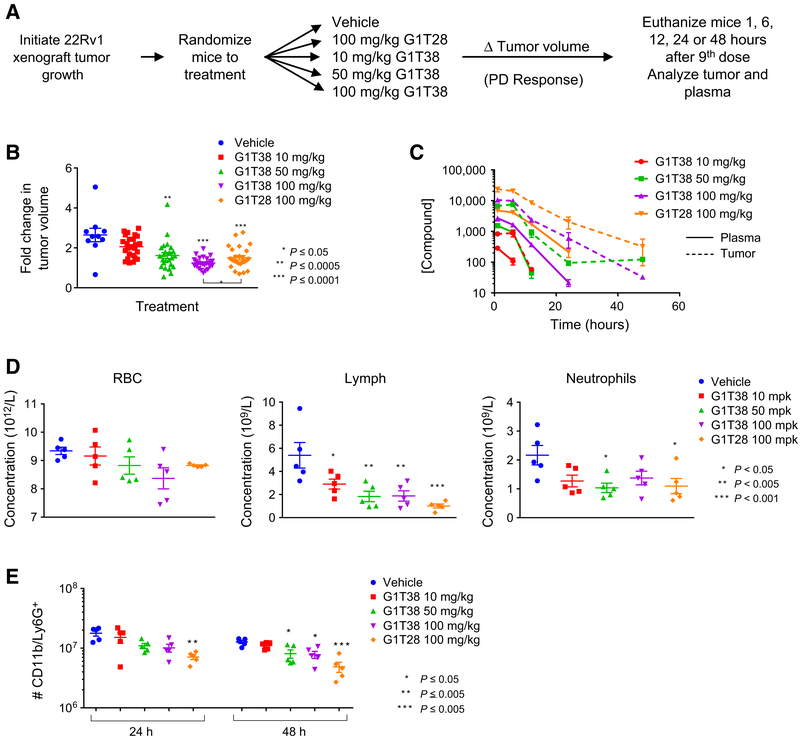
Figure 5.
G1T28 and G1T38 exhibit longer half-life in tumors than in plasma. A, Experimental outline: 22Rv1 xenograft tumors were initiated in castrated male nu/nu mice 12 days prior to the animals being randomized (average tumor volume per group = 0.19 cm3) to treatment with vehicle, G1T28 (100 mg/kg), or G1T38 (10, 50, or 100 mg/kg) orally, every day. Mice were euthanized 1, 6, 12, 24, or 48 hours after the 9th dose (treatment day 8). B, Fold change in tumor volume (with mean and SEM indicated) after 7 days of treatment. ANOVA analysis followed by Bonferroni multiple comparison indicated significant repression of tumor by G1T28 and G1T38 (50 and 100 mg/kg). At equivalent doses (100 mg/kg), G1T38 induced greater tumor growth suppression than did G1T28. C, Drug levels of G1T28 and G1T38 present in tumor tissues (dashed lines) and in plasma (solid lines) 1, 6, 12, 24, or 48 hours after final dose were analyzed by LC/MS-MS. D, ANOVA analysis, followed by Bonferroni multiple comparison test, of CBC counts conducted on whole blood taken from animals euthanized 1 hour after final dose (after 9 days of treatment) revealed no change in red blood cell density (left), reduced levels of lymphocytes (middle), and significant reduction of neutrophil numbers only in animals receiving 50 mg/kg G1T38 and 100 mg/kg G1T28. E, Bone marrow isolated from animals euthanized 24 or 48 hours after the final treatment in the above pharmacokinetic/pharmacodynamic analysis was analyzed by immunostaining (CD11b and Ly6G+) and flow cytometry. Reduced bone marrow neutrophil number (as determined by ANOVA analysis and Bonferroni multiple comparison test) was observed in animals receiving 50 or 100 mg/kg G1T38 or G1T28 (P < 0.05).