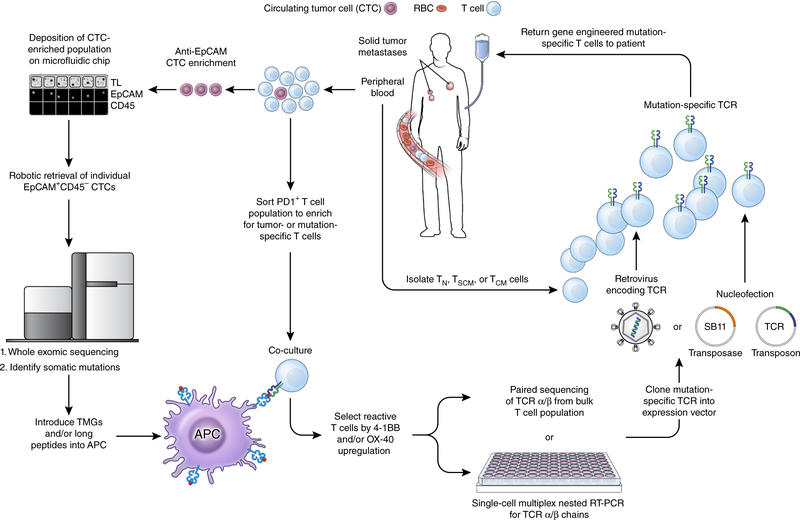
Figure 3.
A pathway for generating autologous TCR gene therapies targeting neoantigens for patients with advanced epithelial cancers. From a single blood draw, all of the requisite components required to produce this therapy can be procured. Circulating tumor cells (CTCs) can be enriched from the blood using a combination of antibody-mediated isolation based on epithelial marker expression (for example, EpCAM) followed by microfluidic isolation. Subsequent genomic extraction, amplification and whole exome sequencing can identify non-synonymous mutations present within the tumor. Circulating T cells that express PD-1 can be isolated and co-cultured with autologous professional antigen presenting cells (APCs) that have either been pulsed with synthetic long peptides harboring the amino acid change resulting from the mutation or RNA-electroporated with tandem minigenes (TMGs) encoding the amino acid change and flanked on either side by 12 amino acids from the endogenous protein. T cells that upregulate the activation markers 4–1BB and/or OX-40 can then be isolated and the α and β chains of the TCR associated with this cell can be sequenced. The α/β TCR that confers reactivity against a neoantigen can then be cloned into an expression vector, for example an integrating virus or the Sleeping Beauty (SB) transposon/transposase system. T cell subsets isolated from the peripheral blood of the patient can finally be modified with this expression vector, expanded in vitro to numbers sufficient for treatment, and re-infused back into the patient. TL, transmitted light; TN, T naive; TSCM, T stem cell memory; TCM, T central memory; SB11, SB transposase 11.