Abstract
The advent of oral direct-acting antiviral agents (DAAs) has dramatically improved the hepatitis C virus (HCV) treatment landscape in the last 4 years, providing cure rates over 95% with a shorter duration of treatment and a very good safety profile. This has enabled access to treatment in nearly all HCV infected patients. The launch of two pangenotypic fixed dose combinations (FDCs) in 2017 made a new step forward in HCV treatment by slightly increasing efficacy and more importantly allowing the treatment of patients without HCV genotyping, and in some cases without fibrosis assessment. However, retreatment of the few DAA failure patients was still an issue for some HCV genotypes. The launch of the triple regimen FDC, sofosbuvir/velpatasvir/voxilaprevir, solves this issue by providing a cure rate over 96% regardless of HCV genotype. In this review, we describe the current HCV treatment landscape and focus on the development of this triple FDC either in treatment-naïve or treatment-experienced patients with previous failure on a DAA regimen.
Keywords: DAA failure, fixed dose combination, glecaprevir, pibrentasvir, single pill tablet, sofosbuvir, velpatasvir, voxilaprevir
Introduction
Hepatitis C virus (HCV) is a hepatotropic RNA virus that causes progressive liver disease that may result in liver cirrhosis and hepatocellular carcinoma. The latest assessment from the World Health Organization suggests that roughly 71 millions people are chronically infected by HCV worldwide.1 The major routes of contamination for this blood-born infection are unsafe drug injections and unsafe medical procedures. Sexual transmission is rare except in men who have sex with men with high-risk sexual behavior.2,3
Until 2011, treatment of chronic HCV hepatitis was based on the use of interferon, at first alone, then in combination with ribavirin (RBV) and later on as a combination of pegylated interferon plus RBV (PR) for 24 or 48 weeks. According to genotype and fibrosis stages, the sustained virological response (SVR) rate with PR varied from 40% up to 70%.4,5 Those treatments were associated with numerous side effects and a deterioration of the quality of life of patients.
Since 2011, several direct-acting antiviral agents (DAAs) have been developed which targeted three proteins involved in different key steps of the HCV life cycle: NS3/4A protease, NS5A protein and NS5B RNA-dependent RNA polymerase. The first DAAs, launched in 2011, were the two NS3/4A protease inhibitors, boceprevir and telaprevir. Combined with PR, those were able to shorten treatment duration and increase the SVR rate, 12 weeks after the completion of treatment, by 30% and up to 70–80%.6–9
Since 2013, other DAAs have been launched, targeting the three proteins. The combination of several DAAs improved the SVR rate to over 90%, with an even shorter duration of treatment at 8, 12, 16 or 24 weeks and a very good safety profile. Patients who could not be treated with PR, such as patients with ongoing intravenous use, severe renal impairments, patients with inherited blood disorders and patients with advanced cirrhosis, were finally able to be treated with those combinations.10–15
Current hepatitis C treatment landscape
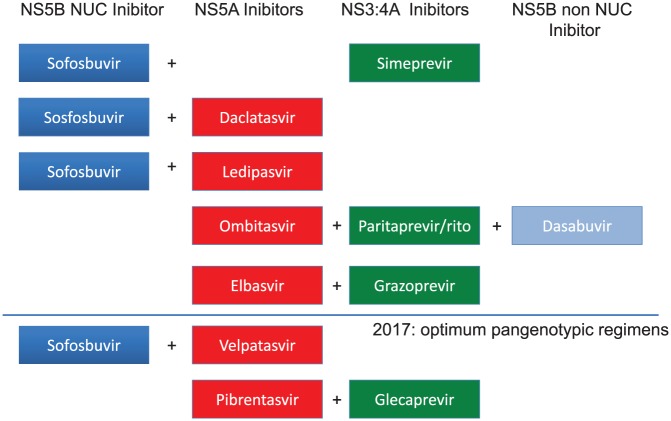
Since 2013, HCV treatment is composed of oral DAA regimens only. Therapies were based on several associations: (1) combinations using sofosbuvir (SOF) the only potent nucleotide NS5B inhibitor as a backbone combined with either protease inhibitor such as simeprevir (SIM) or NS5A inhibitors such as daclatasvir (DCV) or ledipasvir (LDV); (2) a triple combination (PrOD) with a NS3/4A protease inhibitor boosted by ritonavir (paritaprevir) plus a NS5A inhibitor (ombitasvir) plus a non-nucleoside NS5B inhibitor (dasabuvir); and (3) a combination using a potent NS3/4A protease inhibitor, grazoprevir (GZR) plus a second wave NS5A inhibitor, elbasvir (EBR; Figure 1).
Figure 1.
Treatment options with DAA combinations since 2013.
DAA, direct-acting antiviral agent; NUC, nucleotide.
The SVR rate of phase III studies and large real-life data according to genotype and fibrosis stage are reported in Table 1. The first, all oral, DAA combination was SOF and SIM which only achieved high SVR rates in patients with genotype 1 and 4 both in clinical trials and in real-life cohorts.16–21 However, the SVR rates were slightly disappointing in patients with cirrhosis, especially in patients with genotype 1a who harbored a baseline Q80K mutation. The second combination of SOF and DCV was available soon after and was the first pangenotypic combination achieving SVR rates over 90–95% in patients infected by genotypes 1–4, across all studies, excepted in patients with genotype 3 and cirrhosis in whom the SVR rate, with or without RBV, did not achieved the 90% threshold.22–29 The third association available was the triple combination PrOD. This combination achieved a very high SVR rate over 95% in patients with genotype 1, with a lower SVR in patients with cirrhosis or subtype 1a and in those who harbored baseline resistance associated substitutions (RASs).30–37 The double combination of paritaprevir boosted by ritonavir plus ombitasvir (PrO) was also highly potent in patients with genotype 4 in clinical trials and in real-life data.37–39
Table 1.
SVR12 according to genotype and fibrosis stage in phase III trials and large RL studies.
| GT-1 | GT-2 | GT-3 | GT-4 | GT-5/GT-6 | |
|---|---|---|---|---|---|
| SOF + SIM | 97% (F0–F3),16
83–92% (F4)17,18 84% (RL)19 |
100% (F0–F4)20
91–92% (RL)18,21 |
|||
| SOF + DCV | 98% (F0–F4)22
95–98% (RL)23,24 |
92% (F0–F4)22
100% (RL)25 |
96% (F0–F3)26
63–90%**(F4),26 89–92% (RL)23,27 |
95–97% (RL)28,29 | |
| SOF + LDV | 94–99% (F0–F4)13,40–43
91–95% (RL)35,36 |
96% (F0–F4)44 | 89%**(F0–F4)45
78% (RL)36 |
93% (F0–F4)46
97% (RL)36 |
GT-5: 95%47
GT-6: 96%45 GT-6: 95% (RL)48 |
| PrOD | 96%–99% (F0–F3)30–32
91%–96%*(F4)33 92–98% (RL)34–36,37 |
||||
| PrO | 94–98%** (F0–F4)38,39
98–100%** (RL)34,37 |
||||
| GZR/EBR | 92–99% (F0–F4)11,49–53
93–99% (RL)54,55 |
73–80%**56 | 45–57%**(F0–F3)57
100% +SOF (F4)58 |
90%–100%**50,56,59
95–97% (RL)54,55 |
GT-5: 25–100%**56
GT-6: 75–80%50,56 |
24 weeks; ** ± ribavirin.
F0–F3: fibrosis stage 0–3; F4: cirrhosis; RL: real-life; SVR, sustained virological response.
DCV, daclatasvir; EBR, elbasvir; GZR, grazoprevir; LDV, ledipasvir; PrO, paritaprevir boosted by ritonavir plus ombitasvir; PrOD, NS3/4A protease inhibitor boosted by ritonavir (paritaprevir) plus a NS5A inhibitor (ombitasvir) plus a non-nucleoside NS5B inhibitor (dasabuvir); SIM, simeprevir; SOF, sofosbuvir.
Subsequently a second wave of NS5A inhibitors became available with two new, single pill, fixed dose combinations (FDCs). The combination of SOF plus LDV was the first single pill FDC available and was highly potent in patients with genotype 1, 4, 5 and 6 at any fibrosis stage.13,40–43,45–48 This combination was the first one that demonstrated potent activity in patients with genotype 1 and 4 and decompensated cirrhosis.60,61 This combination was suboptimal for patients with genotype 3 infections.45 For patients with genotype 2 infections, a small study demonstrated the potency of this combination in New Zealand but this result was not endorsed by European Medicines Agency (EMA) or the United States Food and Drug Administration (US FDA).44 The FDC of GZR plus EBR was highly potent in patients with genotype 1 and 4 both in studies and in real life, even if patients with subtype 1a and baseline RASs may have needed a longer duration of treatment (16 weeks) in order to achieve the highest SVR rate.49–56,59 The GZR/EBR combination demonstrated a suboptimal SVR rate in patients with genotype 2, 3, 5 and 6.56,57 In contrast, the combination of SOF + GZR/EBR demonstrated a high efficacy rate in patients with genotype 3 and this option was, at least, endorsed by the American Association for the Study of Liver Diseases (AASLD) guidelines but not by European Association for Study of the Liver (EASL) guidelines.58
Since 2017, two pangenotypic FDCs, SOF/velpatasvir (VEL) and glecaprevir (GLE)/pibrentasvir (PIB) were approved and launched by the US FDA and in some of the European Union countries by the EMA. SOF/VEL is the first FDC, given for 12 weeks regardless of genotype and fibrosis stage.62–65 This combination for 12 weeks with RBV achieved a very high SVR rate in patients with decompensated cirrhosis whatever the genotype.14 GLE/PIB offers a FDC, three pills daily, for 8 weeks in naïve patients without cirrhosis regardless of the genotype and for 12 weeks in patients with cirrhosis.66–70 However, patients with genotype 3 and compensated cirrhosis need to be treated for 16 weeks.71 GLE/PIB could not be used in patients with decompensated cirrhosis, due to the presence of NS3/4A protease inhibitors. These treatments provided over 95% of SVR in the phase II and III pivotal studies15 (Table 2).
Table 2.
SVR12 according to genotype and fibrosis stage in phase III trials for pangenotypic combinations14,62–64,68,70,71.
| GT-1 | GT-2 | GT-3 | GT-4 | GT-5 | GT-6 | |
|---|---|---|---|---|---|---|
| SOF/VEL 12 weeks F4dc = SOF/VEL + RBV |
99% (F0–F4) F4dc*: 94% 1a 100%1b |
100% (F0–F4) F4dc*: 100% |
97% (F0–F3) 91% F4 F4dc*: 85% |
100% (F0–F4) F4dc: 100% |
97% (F0–F3) 100% F4 |
100% (F0–F4) |
| GLE/PIB F0–F3: 8w F4: 12w |
99.8% (F0–F3) 99% (F4) |
99% (F0–F3) 100% (F4) |
97% (F0–F3) 96%** (F4) |
100% (F0–F4) |
100% (F0–F4) | 100% (F0–F4) |
SOF/VEL + RBV; ** GLE/PIB for 16 weeks.
F4dc: decompensated cirrhosis; GLE, glecaprevir; PIB, pibrentasvir; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir.
Treatment options in case of DAA treatment failure
Despite the overall high success rate of those new DAA therapies, a small proportion of treated patients did not achieve SVR, mainly due to relapses and, rarely, to viral breakthrough under treatment.72 Several factors may favor DAA failure to the first generation of DAAs, such as cirrhosis, virological factors, genotype 1a and 3 and RASs either pre-existing as natural polymorphisms or induced by a previous DAA regimen. A recent study using the HCV disease burden model (HEP-SIM) suggested that according to the number of patients treated with DAAs between 2014 and 2020 in five European countries (France, Germany, Italy, Spain and the United Kingdom), we can expect to have 47.000 DAA failure patients during this period and nearly all patients treated since 2015 will be NS5A-failures.73 In the guidelines, several retreatment options according to genotype were proposed.
Patients who failed on SOF alone or SOF plus RBV or SOF plus PR can be retreated with several combinations according to the genotype. Most of the combinations may reuse SOF due to the fact that the rare NS5B RASs have a bad fitness and therefore are rarely persistent.15
Patients, who failed the DAA regimen with SOF plus protease inhibitors without NS5A inhibitors, can be retreated with a combination of SOF with NS5A inhibitors (DCV, LDV, VEL).13,22,41,62
In patients with genotype 1 or 4 who failed DAAs regimen containing NS5A inhibitors, four options were available. The first one combined SOF with PrOD for genotype 1 or PrO for genotype 4 plus RBV either for 12 weeks in patients with mild fibrosis or for 24 weeks in patients with subtype 1a and in patients with severe fibrosis or compensated cirrhosis.15 This option achieved an SVR rate of 95%.74 The second option combined SOF with GZR/EBR plus RBV for 12 weeks in patients with mild fibrosis or for 24 weeks in patients with subtype 1a and in patients with severe fibrosis or compensated cirrhosis. Few data demonstrated that this combination was highly effective in achieving SVR in all patients with mild disease treated for 12 weeks and in all patients with NS5A RASs treated for 16 weeks.75,76 The third option combined SOF with DCV and SIM plus RBV for 12 weeks in patients with mild fibrosis or for 24 weeks in patients with subtype 1a and in patients with severe fibrosis or compensated cirrhosis.76 This option was not endorsed by all real-life data.77,78 This combination in DAA failure patients, some with advanced compensated cirrhosis, demonstrated a high rate of adverse (even fatal) events, and a lower rate of response.78 The last option is the association of GLE/PIB plus RBV for 12 or 16 weeks. The study demonstrated a high SVR rate of 96% and over in patients treated either for 12 weeks in those who harbored at baseline only a NS3 RAS or for 16 weeks in those who harbored at baseline only a NS5A RAS. For patients who have both NS3 and NS5A RASs at baseline, the SVR rate was suboptimal.79 Therefore, this combination was not recommended in this situation, at least in the EMA label and in the last EASL guidelines.80
In patients with genotype 2, 3, 5 and 6 who failed a DAA combination with NS5A inhibitors, the recommended retreatment option was the combination of SOF/VEL with RBV for 24 weeks.15 This recommendation was supported by a small multicenter trial in which 69 patients with genotype 1, 2 or 3, who previously failed a NS5A containing DAAs regimen, were retreated with SOF/VEL and RBV for 24 weeks. The SVR rate was 97% in genotype 1 and 93% in genotype two patients regardless of NS5A RASs, but only 78% in patients with genotype 3.81 Therefore there was an urgent need for a pangenotypic rescue regimen for patients who failed previous NS5A-containing DAAs regimens.
Sofosbuvir/velpatasvir/voxilaprevir single pill daily regimen
The preclinical development of SOF, VEL and voxilaprevir (VOX) has been run separately during the last decade.
US FDA and EMA have approved SOF for pangenotypic HCV treatment either in combination with PR or in an interferon-free combination either with RBV or with other DAAs.82 SOF has a high barrier to resistance making it the ideal candidate to be used in combination with other classes of DAA and for retreatment of DAA failure patients.
VEL is a pangenotypic HCV NS5A inhibitor with antiviral activity against the HCV replicon in genotypes 1 through 6.83 Early clinical data supported clinical development with a single dose of 100 mg once daily.84 VEL has a higher barrier to resistance in comparison with the previous NS5A inhibitors, LDV or DCV.
VOX (or GS-9857) is a NS3/4A inhibitor with potent in vitro activity against the HCV genotype 1–6 and an improved resistance profile against the commonly encountered genotype 1 NS3 RASs in comparison with other protease inhibitors.85
Phase II trials
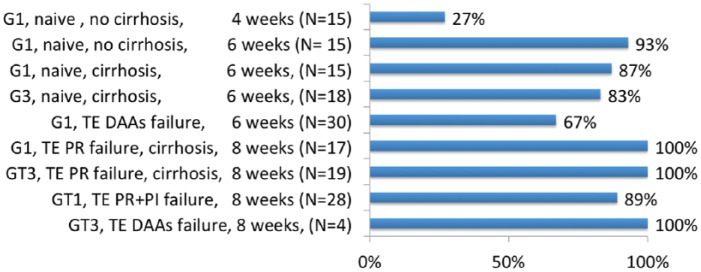
The first phase II study, evaluated FDC SOF/VEL plus VOX 100 mg once daily with food in 161 patients with genotype 1 or 3 for a short treatment duration 4, 6 or 8 weeks86 (Figure 2). The SVR rate was poor (27%) with the 4-week regimen in treatment-naïve patients with genotype 1 and without cirrhosis. The 6-week regimen was associated with a more optimal SVR rate ranging from 67% in patients with genotype 1 who had previously failed a DAA-containing regimen, to 93% in treatment-naïve patients with genotype 1 and without cirrhosis. The 8-week regimen was associated with an optimal SVR rate over 90% whatever the genotype or treatment history. The safety profile of the combination was good with headaches in 23% and diarrhea in 11% of patients. Overall the relapse rates were 19% in those treated for 6 weeks and 4% in those treated for 8 weeks. Moreover, the SVR rates were similar between patients who did and did not harbor baseline RASs. Only two patients had emergent RASs at the time of failure, confirming the high barrier to resistance of this regimen and suggesting the potential of this combination as a salvage regimen of DAA failure.
Figure 2.
SVR in phase II studies with sofosbuvir/velpatasvir plus voxilaprevir.86
DAA, direct-acting antiviral agent; PI, protease inhibitors; PR, pegylated interferon plus ribavirin; SVR, sustained virological response; TE, treatment-experienced.
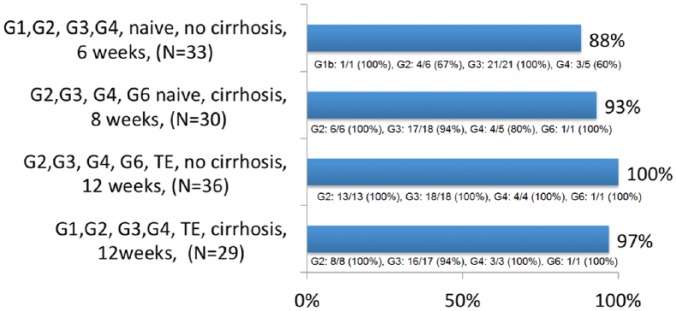
The second phase II study evaluated the same combination SOF/VEL plus VOX 100 mg once daily in 128 HCV genotype 2, 3, 4 or 6 naïve or treatment-experienced HCV patients for various treatment durations of 6, 8 or 12 weeks.87 The 6-week regimen achieved a suboptimal overall SVR rate of 88% in treatment-naïve patients without cirrhosis especially in those with genotype 2 and 4. In contrast, the 8-week regimen achieved a good SVR rate of 93% in treatment-naïve patients with cirrhosis. The 12-week regimen achieved an optimal SVR rate in treatment-experienced patients, including previous DAA failure (38 patients), ranging from 97% in patients with cirrhosis to 100% in patients without cirrhosis (Figure 3). One patient with genotype 3 and cirrhosis had a relapse with treatment-emergent NS3 RAS Q80R that does not confer in vitro resistance to VOX. The safety profile of the triple combination was good. Again, the SVR rates were similar between patients with or without baseline RASs, 92% versus 94% for naïve patients with cirrhosis treated for 8 weeks. In conclusion the triple combination appeared to be a well-tolerated and effective treatment in HCV patients of all genotypes with or without compensated cirrhosis.
Figure 3.
SVR in phase II studies with sofosbuvir/velpatasvir plus voxilaprevir.87
SVR, sustained virological response; TE, treatment-experienced.
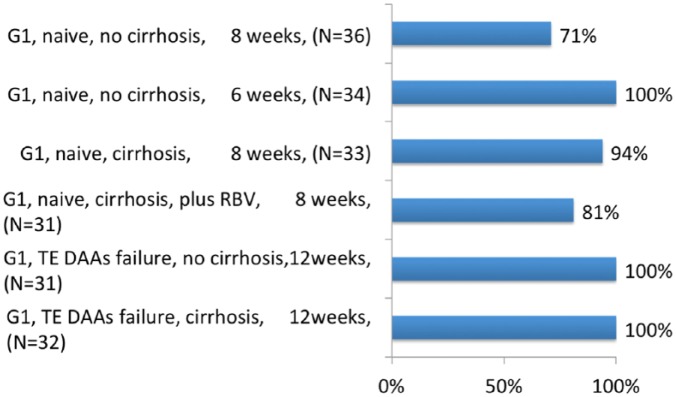
The third phase II study evaluated the same combination of SOF/VEL plus VOX 100 mg once daily among 197 patients with genotype 1 for a 6–12 week treatment duration, plus RBV for treatment-naïve patients with cirrhosis.88 In treatment-naïve patients without cirrhosis, 6 weeks of treatment achieved a suboptimal SVR rate (71%). In contrast, 8 weeks of treatment achieved an SVR in all patients. In treatment-naïve patients with cirrhosis, 8 weeks of treatment achieved an SVR rate of 87.5% with no benefit of RBV addition, 81% versus 94%. In treatment-experienced patients who previously failed a DAA regimen (46% with NS5A inhibitors, 54% with NS3/4A protease inhibitors and 39% with SOF) all patients with or without cirrhosis achieved SVR after 12 weeks of treatment (Figure 4). Overall, 18 patients relapsed and no patient experienced breakthrough. Overall, one patient died during follow up from atypical pneumonia. In this study the triple combination appeared well tolerated and effective for 8 weeks in treatment-naïve patients with no benefit of RBV addition. A 12-week treatment duration achieved an SVR in all 63 DAA-experienced patients with genotype 1.
Figure 4.
SVR in phase II studies with sofosbuvir/velpatasvir plus voxilaprevir.88
DAA, direct-acting antiviral agent; RBV, ribavirin; SVR, sustained virological response; TE, treatment-experienced.
The last phase II study evaluated the same combination SOF/VEL plus VOX 100 mg once daily with or without RBV for 12 weeks in 49 patients with genotype 1 who previously failed a DAA regimen.89 A total of 51% of patients had compensated cirrhosis at baseline and 12% of patients had already failed a 2 or 3 DAA regimen. All patients treated without RBV achieved SVR and 24 out 25 patients (96%) treated with RBV achieved SVR. Baseline RASs were present at baseline in 73% of cases and SVR was not different according to the presence of RASs (97% versus 100%). The only patient who relapsed was a black male with cirrhosis previously treated by SOF/LDV for 24 weeks. He had developed new RASs at relapse in both NS5A and NS3 domain. Overall this study demonstrated that 12 weeks of SOF/VEL/VOX was effective and well tolerated among patients with genotype 1 HCV who had previously failed a DAA-based regimen. Moreover, the potency of this regimen can obviate the need for RBV to be included in the regimen to maximize efficacy.
Phase III trials: the POLARIS studies
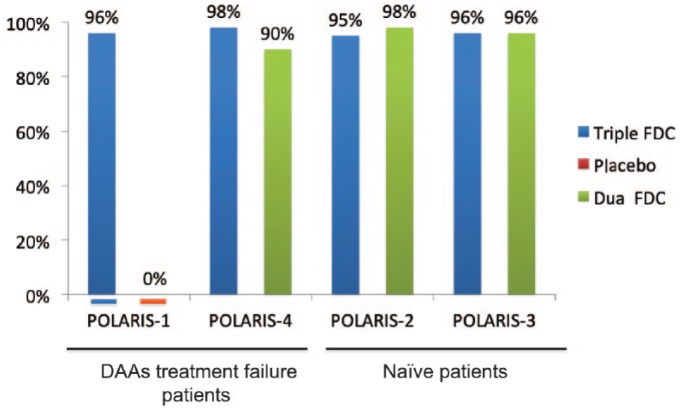
The phase III program had evaluated the triple FDC SOF/VEL/VOX for 8 weeks in treatment-naïve patients of all genotypes and for 12 weeks in patients of all genotypes who have received previous treatment with any DAA (Figure 5).
Figure 5.
SVR in phase III studies with sofosbuvir/velpatasvir plus voxilaprevir.90,91,92
DAA, direct-acting antiviral agent; FDC, fixed dose combination; SVR, sustained virological response.
Naïve or treatment-experienced without DAA patients
The POLARIS 2 and 3 studies assessed the efficacy of 8 weeks of treatment with the triple FDC in HCV patients either naïve of treatment or treatment-experienced without DAAs in patients with or without compensated cirrhosis.90 Patients were assigned randomly in groups and given triple FDCs for 8 weeks or dual FDCs (SOF/VEL) for 12 weeks.
POLARIS-2, enrolled patients infected with all HCV genotypes with or without cirrhosis, except patients with genotype 3 and cirrhosis. It was designed to test non-inferiority of 8 weeks of triple FDCs to 12 weeks of dual FDCs using a non-inferiority margin of 5%. A total of 941 patients began treatment, 77% were treatment-naïve, and 18.5% had compensated liver cirrhosis. Overall, 95% [95% confidence interval (CI), 93–97%] of patients had an SVR with 8 weeks of triple FDCs; this did not meet the criteria to establish non-inferiority to 12 weeks of dual FDCs, which produced an SVR in 98% of patients (95% CI, 96–99%; difference in the stratum-adjusted Mantel–Haenszel proportions of −3.2% 95% CI, −6.0 to 0.4%). The difference in the efficacy was primarily due to a lower rate of SVR (92%) among US patients with HCV genotype 1a infection receiving 8 weeks of triple FDCs. The relapse rate was observed in 4% of patients treated with triple FDCs for 8 weeks versus 1% in those treated with dual FDCs for 12 weeks. Baseline RASs to NS3 or NS5A inhibitors were found in 50% of patients. Of these, 94% had an SVR as compared with 98% for patients without RASs. Baseline Q80K RAS nevertheless was associated with a reduction in SVR rate for genotype 1a patients receiving a triple FDC regimen for 8 weeks, 88% with Q80K compared with 94% without. Only one patient had treatment-emergent NS5A RAS Q30R and L31M.
POLARIS-3, which enrolled patients with genotype 3 HCV infections and cirrhosis, compared rates of SVR between a group treated with 8 weeks of triple FDCs and another group treated with 12 weeks of dual FDCs with a performance goal of 83%. A total of 219 patients began treatment, 69% of them were treatment-naïve. Overall, 96% of patients achieved an SVR in both treatment groups, which was significantly superior to the performance goal that was based on the prior results of this dual therapy in this patients population in the ASTRAL-3 trial (SVR, 91%; 95% CI, 83–96).63 Among the 67 treatment-experienced patients, the SVR rate was numerically higher in patients treated with triple FDCs compared with those treated with dual FDCs (97% versus 91%). All 46 patients with baseline RASs achieved an SVR. Neither of the two patients who relapsed after triple FDCs for 8 weeks had treatment-emergent RAS.
None of the 611 patients receiving 8 weeks of triple FDCs in both studies discontinued treatment owing to adverse events. Less than 3% of patients had serious adverse events, and one patient died during the follow up. The safety profile was fine. The most common adverse events with triple FDCs were headache (26%), fatigue (22%), diarrhea (17%) and nausea (16%). Mild gastrointestinal adverse events were associated with the regimen including VOX. Moreover in these two phase III studies, patient-reported outcomes (PROs) were collected.93 During treatment, improvements in most PRO scores were significant. After treatment discontinuation, patients treated with both regimens achieved significant and clinically meaningful PRO gains.
Treatment-experienced DAA patients
The POLARIS 1 and 4 studies assessed the efficacy of 12 weeks of treatment with the triple FDCs in DAA treatment-experienced HCV patients with or without compensated cirrhosis.91
POLARIS-1 enrolled 415 patients, infected with any HCV genotype, who previously failed a regimen containing an NS5A inhibitor. Patients with genotype 1 were randomly assigned in a 1:1 ratio to receive either the triple FDC (150 patients) or matching placebo (150 patients) once daily for 12 weeks. Patients who were infected by other genotypes (114 patients) were enrolled in the triple FDC once daily for 12 weeks. Of these patients, one patient with genotype 4 never received treatment. A total of 46% of patients had compensated cirrhosis. The most common NS5A inhibitors used in a previous unsuccessful treatment were LDV (55%), DCV (23%) and ombitasvir (13%). Overall, 39% of patients had received at least two or more previous HCV treatments. The rate of SVR was 96% compared with 0% with placebo. Overall the rate of SVR was 99% among patients without cirrhosis and 93% among patients with cirrhosis. According to HCV genotype, all patients with genotype 1b, 2, 5 and 6 achieved an SVR. The SVR rate was 96% in patients with genotype 1a, 95% in patients with genotype 3 and 91% in genotype 4. Baseline RASs were present at baseline in 83% of patients and 79% harbored NS5A RASs. The SVR rate was similar between patients with baseline RASs (96%) as compared with those without RASs (99%). A total of six patients with cirrhosis had a relapse (one patient with genotype 1a, four patients with genotype 3 and one patient with genotype 4). Overall, one patient with genotype 4 had treatment-emergent RASs and one patient had a breakthrough during treatment with low plasma concentration of the drug on treatment, suggestive of nonadherence.
A total of 147 genotype 1 patients who received placebo were subsequently treated with triple FDCs once daily for 12 weeks.92 Overall, one-third of patients had cirrhosis and 77% of them were genotype 1a. Overall the SVR rate was 97%. Patients with cirrhosis had an SVR rate of 98% and those without cirrhosis had an SVR rate of 97%. The SVR rate was 97% for patients with baseline RASs and 100% in those without baseline RASs. Overall, four patients with genotype 1a, one with cirrhosis, experienced relapse. All had baseline RASs and two developed treatment-emergent RASs. Combining the data in the primary and sub-study of POLARIS-1, the overall SVR rate was 97% (396/409).
POLARIS-4 enrolled 333 patients who previously failed a DAA regimen without an NS5A inhibitor. HCV patients with genotype 1, 2 and 3 were randomly assigned in a 1:1 ratio to receive triple FDCs (163 patients) or dual FDCs (151 patients) for 12 weeks. An additional 19 patients with genotype 4 were enrolled in the triple FDC regimen for 12 weeks.91 A total of 46% of patients had compensated cirrhosis. Overall, 85% of patients had received SOF as part of the previous regimen and 26% had received NS3/4A inhibitors. A total of 39% of patients had received at least two or more previous HCV treatments. Overall, the rate of SVR was 98% in patients receiving triple FDCs and 90% in those receiving dual FDCs. Among patients without cirrhosis the rate of SVR was 98% among those receiving triple FDCs and 94% among those receiving dual FDCs as compared with 98% and 86% respectively among patients with cirrhosis. According to genotype, the SVR rate was, for those receiving triple FDCs as compared with those receiving dual FDCs, in patients with genotype 1a, 98% versus 89%, in patients with genotype 1b, 96% versus 95%, in patients with genotype 2, 100% versus 97%, and in patients with genotype 3, 96% versus 85% respectively. A total of 49% of enrolled patients had baseline RASs to NS3 or NS5A inhibitors. Only 1 patient relapsed in those treated with triple FDCs as compared with 14 patients in those treated with dual FDCs. The patient who failed on triple FDCs had no baseline RASs and no treatment-emergent RASs.
The safety profile in the POLARIS-1 and 4 studies was good. The percentage of patients who discontinued treatment due to adverse events was 1% or lower. The most common adverse events with triple FDCs were headache, fatigue, diarrhea and nausea.
Moreover, in these two phase III studies, PROs were collected.94 After 12 weeks of treatment some PRO scores improved in both the dual and triple FDC treatment groups but not in the placebo group. All increases in PRO scores were sustained or increased after the end of the treatment. There was no difference in PROs between dual or triple FDCs. These findings indicate the benefit of these regimens during treatment and after SVR.
Regulatory issues and guidelines recommendations
Results of the phase III studies lead to different approvals from US FDA and EMA.
On 18 July 2017, the US FDA approved SOF/VEL/VOX FDC for 12 weeks for adult HCV patients with or without compensated cirrhosis (Child–Pugh A) and any genotypes that have previously failed an HCV regimen containing an NS5A inhibitor. This regimen is also approved in patients with genotype 1a or 3 with or without compensated cirrhosis who have previously failed an HCV regimen containing SOF without a NS5A inhibitor.95 Those approvals were implemented in the recent AASLD guidelines.
In contrast on 27 July 2017, the EMA approved SOF/VEL/VOX FDC with wider indications: 8 weeks of FDCs, any genotype, in treatment-naïve patients without cirrhosis and in patients with genotype 3 with cirrhosis; 12 weeks of FDCs, any genotype, in treatment-naïve patients with cirrhosis and, in treatment-experienced patients with DAA failures with or without compensated cirrhosis.
The last EASL guidelines have implemented few of the EMA approvals. FDC is recommended for 12 weeks in patients with genotype 3 with cirrhosis, either treatment-naïve or experienced. FDCs for 12 weeks is the first-line treatment recommended in patients with or without compensated cirrhosis who failed a previous regimen with DAAs, either a protease inhibitor or NS5A inhibitor. An alternative option is the combination of SOF plus GLE/PIB ± RBV for 12–16 weeks based on two small studies involving patients who failed previous DAA regimens, including the GLE/PIB regimen.96,97 The SVR rate was 96%.96 In very difficult-to-cure patients with NS5A RASs who failed twice to achieve an SVR after several DAA regimens, including protease and N5A inhibitors, FDCs are recommended to be used either in combination with weight-based dose RBV or to extend the treatment duration to 16 or 14 weeks.
Conclusion
The FDC of SOF/VEL/VOX is a well-tolerated pangenotypic, once daily, single tablet regimen. The 8-week treatment has shown a very high efficacy, almost similar to the dual FDC SOF/VEL regimen for 12 weeks or the GLE/PIB regimen for 8 weeks in treatment-naïve patients or treatment-experienced without DAA patients, without cirrhosis of any genotype. In patients with compensated cirrhosis, 8 weeks of triple FDCs have shown similar efficacy as the dual FDCs for 12 weeks, except for patients with genotype 1a and cirrhosis. The EMA proposes to use triple FDCs for 12 weeks in patients with compensated cirrhosis with the exception of patients with genotype 3, for which strong data from POLARIS-3 demonstrated the efficacy of 8-week regimen. In DAA treatment-experienced patients, with or without compensated cirrhosis, 12 weeks of triple FDCs achieved a very high efficacy, even in patients with baseline NS5A RASs.
This triple FDC is a hallmark therapeutic achievement in HCV therapy for any genotype, fibrosis stage or previous DAA failure. One pill that fits all is therefore nearly achievable. In combination with other pangenotypic options available, such as dual combination (SOF/VEL or GLE/PIB) or triple combination SOF plus GLE/PIB, we can expect to be near the end of the HCV cure road.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The following are declared: M. Bourlière: advisory board and speaker for: Gilead, AbbVie, MSD, BMS, Janssen, Boehringer-Ingelheim; Paul Castellani: speaker for Gilead, AbbVie, Janssen, and MSD; Valérie Oules: speaker for Gilead, AbbVie, Janssen, MSD; Xavier Adhoute: speaker for Bayer.
Contributor Information
Marc Bourlière, Hepato-Gastroenterology Department, Hospital Saint Joseph, 26 Bd de Louvain 13008 Marseilles, France.
Olivia Pietri, Hepato-Gastroenterology Department, Hospital Saint Joseph, Marseilles, France.
Paul Castellani, Hepato-Gastroenterology Department, Hospital Saint Joseph, Marseilles, France.
Valérie Oules, Hepato-Gastroenterology Department, Hospital Saint Joseph, Marseilles, France.
Xavier Adhoute, Hepato-Gastroenterology Department, Hospital Saint Joseph, Marseilles, France.
References
- 1. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2: 161–176. [DOI] [PubMed] [Google Scholar]
- 2. Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. Aids 2017; 31: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 3. Ingiliz P, Martin TC, Rodger A, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol 2017; 66: 282–287. [DOI] [PubMed] [Google Scholar]
- 4. McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361: 580–593. [DOI] [PubMed] [Google Scholar]
- 5. Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med 2007; 357: 124–134. [DOI] [PubMed] [Google Scholar]
- 6. Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405–2416. [DOI] [PubMed] [Google Scholar]
- 8. Poordad F, McCone J, Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364: 2417–2428. [DOI] [PubMed] [Google Scholar]
- 10. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to treat Hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165: 625–634. [DOI] [PubMed] [Google Scholar]
- 11. Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naïve and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 2015; 386: 1537–1545. [DOI] [PubMed] [Google Scholar]
- 12. Hezode C, Colombo M, Bourliere M, et al. Elbasvir/Grazoprevir for patients with hepatitis C virus infection and inherited blood disorders: a phase III study. Hepatology 2017; 66: 736–745. [DOI] [PubMed] [Google Scholar]
- 13. Bourliere M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015; 15: 397–404. [DOI] [PubMed] [Google Scholar]
- 14. Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015; 373: 2618–2628. [DOI] [PubMed] [Google Scholar]
- 15. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66: 153–194. [DOI] [PubMed] [Google Scholar]
- 16. Kwo P, Gitlin N, Nahass R, et al. Simeprevir plus sofosbuvir (12 and 8 Weeks) in HCV genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology 2016; 64; 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawitz E, Matusow G, DeJesus E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2). Hepatology 2016; 64: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marino Z, Pascasio-Acevedo JM, Gallego A, et al. High efficacy of Sofosbuvir plus Simeprevir in a large cohort of Spanish cirrhotic patients infected with genotypes 1 and 4. Liver Int 2017; 37: 1823–1832. [DOI] [PubMed] [Google Scholar]
- 19. Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology 2016; 150: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buti M, Calleja JL, Lens S, et al. Simeprevir in combination with sofosbuvir in treatment-naive and -experienced patients with hepatitis C virus genotype 4 infection: a phase III, open-label, single-arm study (PLUTO). Aliment Pharmacol Ther 2017; 45: 468–475. [DOI] [PubMed] [Google Scholar]
- 21. Willemse SB, Baak LC, Kuiken SD, et al. Sofosbuvir plus simeprevir for the treatment of HCV genotype 4 patients with advanced fibrosis or compensated cirrhosis is highly efficacious in real life. J Viral Hepat 2016; 23: 950–954. [DOI] [PubMed] [Google Scholar]
- 22. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370: 211–221. [DOI] [PubMed] [Google Scholar]
- 23. Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut 2016; 65: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pol S, Bourliere M, Lucier S, et al. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J Hepatol 2017; 66: 39–47. [DOI] [PubMed] [Google Scholar]
- 25. Mangia A, Arleo A, Copetti M, et al. The combination of daclatasvir and sofosbuvir for curing genotype 2 patients who cannot tolerate ribavirin. Liver Int 2016; 36: 971–976. [DOI] [PubMed] [Google Scholar]
- 26. Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015; 61: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hezode C, Lebray P, De Ledinghen V, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a French early access programme. Liver Int 2017; 37: 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yakoot M, Abdo AM, Abdel-Rehim S, et al. Response tailored protocol versus the fixed 12weeks course of dual sofosbuvir/daclatasvir treatment in egyptian patients with chronic hepatitis C genotype-4 infection: a randomized, open-label, non-inferiority trial. EBioMedicine 2017; 21: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Omar H, El Akel W, Elbaz T, et al. Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther 2018; 47: 421–431. [DOI] [PubMed] [Google Scholar]
- 30. Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370: 1604–1614. [DOI] [PubMed] [Google Scholar]
- 31. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 32. Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 2014; 147: 359–365, e1. [DOI] [PubMed] [Google Scholar]
- 33. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 34. Wedemeyer H, Craxi A, Zuckerman E, et al. Real-world effectiveness of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: a meta-analysis. J Viral Hepat 2017; 24: 936–943. [DOI] [PubMed] [Google Scholar]
- 35. Backus LI, Belperio PS, Shahoumian TA, et al. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther 2017; 22: 481–493. [DOI] [PubMed] [Google Scholar]
- 36. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the veterans affairs national health care system. Gastroenterology 2016; 151: 457–471, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perello C, Carrion JA, Ruiz-Antoran B, et al. Effectiveness and safety of ombitasvir, paritaprevir, ritonavir +/− dasabuvir +/− ribavirin: an early access programme for Spanish patients with genotype 1/4 chronic hepatitis C virus infection. J Viral Hepat 2017; 24: 226–237. [DOI] [PubMed] [Google Scholar]
- 38. Asselah T, Hezode C, Qaqish RB, et al. Ombitasvir, paritaprevir, and ritonavir plus ribavirin in adults with hepatitis C virus genotype 4 infection and cirrhosis (AGATE-I): a multicentre, phase 3, randomised open-label trial. Lancet Gastroenterol Hepatol 2016; 1: 25–35. [DOI] [PubMed] [Google Scholar]
- 39. Waked I, Shiha G, Qaqish RB, et al. Ombitasvir, paritaprevir, and ritonavir plus ribavirin for chronic hepatitis C virus genotype 4 infection in Egyptian patients with or without compensated cirrhosis (AGATE-II): a multicentre, phase 3, partly randomised open-label trial. Lancet Gastroenterol Hepatol 2016; 1: 36–44. [DOI] [PubMed] [Google Scholar]
- 40. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- 41. Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370: 1483–1493. [DOI] [PubMed] [Google Scholar]
- 42. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370: 1879–1888. [DOI] [PubMed] [Google Scholar]
- 43. Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 2015; 62: 79–86. [DOI] [PubMed] [Google Scholar]
- 44. Gane EJ, Hyland RH, Yang Y, et al. Efficacy of ledipasvir plus sofosbuvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2 infection. Gastroenterology 2017; 152: 1366–1371. [DOI] [PubMed] [Google Scholar]
- 45. Gane EJ, Hyland RH, An D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015; 149: 1454–1461, e1. [DOI] [PubMed] [Google Scholar]
- 46. Abergel A, Metivier S, Samuel D, et al. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology 2016; 64: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 47. Abergel A, Asselah T, Metivier S, et al. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. The Lancet infectious diseases 2016; 16: 459–464. [DOI] [PubMed] [Google Scholar]
- 48. Wong RJ, Nguyen MT, Trinh HN, et al. Community-based real-world treatment outcomes of sofosbuvir/ledipasvir in Asians with chronic hepatitis C virus genotype 6 in the United States. J Viral Hepat 2017; 24: 17–21. [DOI] [PubMed] [Google Scholar]
- 49. Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 2015; 385: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 50. Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015; 163: 1–13. [DOI] [PubMed] [Google Scholar]
- 51. Kwo P, Gane EJ, Peng CY, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology 2017; 152: 164–175 e4. [DOI] [PubMed] [Google Scholar]
- 52. Sperl J, Horvath G, Halota W, et al. Efficacy and safety of elbasvir/grazoprevir and sofosbuvir/pegylated interferon/ribavirin: a phase III randomized controlled trial. J Hepatol 2016; 65: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 53. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis C virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology 2017; 152: 1372–1382 e2. [DOI] [PubMed] [Google Scholar]
- 54. Flamm SL, Bacon B, Curry MP, et al. Real-world use of elbasvir-grazoprevir in patients with chronic hepatitis C: retrospective analyses from the TRIO network. Aliment Pharmacol Ther 2018; 47: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 55. Kramer JR, Puenpatom A, Erickson KF, et al. Real-world effectiveness of elbasvir/grazoprevir In HCV-infected patients in the US veterans affairs healthcare system. J Viral Hepat. Epub ahead of print 31 May 2018. DOI: 10.1111/jvh.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brown A, Hezode C, Zuckerman E, et al. Efficacy and safety of 12 weeks of elbasvir +/− grazoprevir +/− ribavirin in participants with hepatitis C virus genotype 2, 4, 5 or 6 infection: the C-SCAPE study. J Viral Hepat 2018; 25: 457–464. [DOI] [PubMed] [Google Scholar]
- 57. Gane E, Nahass R, Luketic V, et al. Efficacy of 12 or 18 weeks of elbasvir plus grazoprevir with ribavirin in treatment-naive, noncirrhotic HCV genotype 3-infected patients. J Viral Hepat 2017; 24: 895–899. [DOI] [PubMed] [Google Scholar]
- 58. Foster GR, Agarwal K, Cramp ME, et al. Elbasvir/grazoprevir and sofosbuvir for HCV genotype 3 infection with compensated cirrhosis: a randomized trial. Hepatology 2018; 67: 2113–2116. [DOI] [PubMed] [Google Scholar]
- 59. Asselah T, Reesink H, Gerstoft J, et al. Efficacy of elbasvir and grazoprevir in participants with hepatitis C virus genotype 4 infection: a pooled analysis. Liver Int 2018; 38: 1583–1591. [DOI] [PubMed] [Google Scholar]
- 60. Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149: 649–659. [DOI] [PubMed] [Google Scholar]
- 61. Manns M, Samuel D, Gane EJ, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016; 16: 685–697. [DOI] [PubMed] [Google Scholar]
- 62. Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015; 373: 2599–2607. [DOI] [PubMed] [Google Scholar]
- 63. Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373: 2608–2617. [DOI] [PubMed] [Google Scholar]
- 64. Asselah T, Bourgeois S, Pianko S, et al. Sofosbuvir/velpatasvir in patients with hepatitis C virus genotypes 1–6 and compensated cirrhosis or advanced fibrosis. Liver Int 2018; 38: 443–450. [DOI] [PubMed] [Google Scholar]
- 65. Esteban R, Pineda JA, Calleja JL, et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in patients with HCV genotype 3 infection and cirrhosis. Gastroenterology 2018; 155: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 66. Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018; 378: 354–369. [DOI] [PubMed] [Google Scholar]
- 67. Rockstroh JK, Lacombe K, Viani RM, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients co-infected with hepatitis C virus and human immunodeficiency virus-1: the EXPEDITION-2 study. Clin Infect Dis 2018; 73: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Puoti M, Foster GR, Wang S, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir: integrated analysis of HCV genotype 1–6 patients without cirrhosis. J Hepatol 2018; 69: 293–300. [DOI] [PubMed] [Google Scholar]
- 69. Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med 2017; 377: 1448–1455. [DOI] [PubMed] [Google Scholar]
- 70. Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017; 17: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 71. Wyles D, Poordad F, Wang S, et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: a partially randomized phase 3 clinical trial. Hepatology 2018; 67: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Buti M, Riveiro-Barciela M, Esteban R. Management of direct-acting antiviral agent failures. J Hepatol 2015; 63: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 73. Chhatwal J, Chen Q, Ayer T, et al. Hepatitis C virus re-treatment in the era of direct-acting antivirals: projections in the USA. Aliment Pharmacol Ther 2018; 47: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Poordad F, Bennett M, Sepe TE, et al. Ombitasvir/paritaprevir/R, dasabuvir and sofosbuvir treatment of patients with HCV genotype 1infection who failed a prior course of DAA therapy: the QUARTZ-1 study. J Hepatol 2016; 64: S767–S768. [Google Scholar]
- 75. Lawitz E, Poordad F, Gutierrez JA, et al. Short-duration treatment with elbasvir/grazoprevir and sofosbuvir for hepatitis C: a randomized trial. Hepatology 2017; 65: 439–450. [DOI] [PubMed] [Google Scholar]
- 76. de Ledinghen V, Laforest C, Hezode C, et al. Retreatment with Sofosbuvir plus grazoprevir/elbasvir plus ribavirin of patients with hepatitis C virus genotype 1 or 4 who previously failed an NS5A- or NS3-containing regimen: the ANRS HC34 REVENGE study. Clin Infect Dis 2018; 66: 1013–1018. [DOI] [PubMed] [Google Scholar]
- 77. Abdel-Moneim A, Aboud A, Abdel-Gabbar M, et al. A sofosbuvir-based quadruple regimen is highly effective in HCV type 4-infected Egyptian patients with DAA treatment failure. J Hepatol 2018; 68: 1313–1315. [DOI] [PubMed] [Google Scholar]
- 78. Hezode C, Fourati S, Chevaliez S, et al. Sofosbuvir-daclatasvir-simeprevir plus ribavirin in direct-acting antiviral-experienced patients with hepatitis C. Clin Infect Dis 2017; 64: 1615–1618. [DOI] [PubMed] [Google Scholar]
- 79. Poordad F, Pol S, Asatryan A, et al. Glecaprevir/pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure. Hepatology 2018; 67: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69: 461–511. [DOI] [PubMed] [Google Scholar]
- 81. Gane EJ, Shiffman ML, Etzkorn K, et al. Sofosbuvir-velpatasvir with ribavirin for 24 weeks in hepatitis C virus patients previously treated with a direct-acting antiviral regimen. Hepatology 2017; 66: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 82. Kirby BJ, Symonds WT, Kearney BP, et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet 2015; 54: 677–690. [DOI] [PubMed] [Google Scholar]
- 83. Lawitz E, Freilich B, Link J, et al. A phase 1, randomized, dose-ranging study of GS-5816, a once-daily NS5A inhibitor, in patients with genotype 1–4 hepatitis C virus. J Viral Hepat 2015; 22: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 84. Mogalian E, German P, Kearney BP, et al. Preclinical pharmacokinetics and first-in-human pharmacokinetics, safety, and tolerability of velpatasvir, a pangenotypic hepatitis C virus NS5A inhibitor, in healthy subjects. Antimicrob Agents Chemother 2017; 61: e02084–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rodriguez-Torres M, Glass S, Hill J, et al. GS-9857 in patients with chronic hepatitis C virus genotype 1–4 infection: a randomized, double-blind, dose-ranging phase 1 study. J Viral Hepat 2016; 23: 614–622. [DOI] [PubMed] [Google Scholar]
- 86. Gane EJ, Schwabe C, Hyland RH, et al. Efficacy of the combination of sofosbuvir, velpatasvir, and the NS3/4A protease inhibitor GS-9857 in treatment-naive or previously treated patients with hepatitis C virus genotype 1 or 3 infections. Gastroenterology 2016; 151: 448–456, e1. [DOI] [PubMed] [Google Scholar]
- 87. Gane EJ, Kowdley KV, Pound D, et al. Efficacy of sofosbuvir, velpatasvir, and GS-9857 in patients with hepatitis C virus genotype 2, 3, 4, or 6 infections in an open-label, phase 2 trial. Gastroenterology 2016; 151: 902–909. [DOI] [PubMed] [Google Scholar]
- 88. Lawitz E, Reau N, Hinestrosa F, et al. Efficacy of sofosbuvir, velpatasvir, and GS-9857 in patients with genotype 1 hepatitis C virus infection in an open-label, phase 2 trial. Gastroenterology 2016; 151: 893–901, e1. [DOI] [PubMed] [Google Scholar]
- 89. Lawitz E, Poordad F, Wells J, et al. Sofosbuvir-velpatasvir-voxilaprevir with or without ribavirin in direct-acting antiviral-experienced patients with genotype 1 hepatitis C virus. Hepatology 2017; 65: 1803–1809. [DOI] [PubMed] [Google Scholar]
- 90. Jacobson IM, Lawitz E, Gane EJ, et al. Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology 2017; 153: 113–122. [DOI] [PubMed] [Google Scholar]
- 91. Bourliere M, Gordon SC, Flamm SL, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med 2017; 376: 2134–2146. [DOI] [PubMed] [Google Scholar]
- 92. Bourliere M, Gordon SC, Schiff ER, et al. Sofosbuvir-velpatasvir-voxilaprevir after blinded placebo in NS5A-inhibitor-experienced patients with chronic hepatitis C in the phase 3 POLARIS-1 study. Lancet Gastroenterol Hepatol. Epub ahead of print 31 May 2018. DOI: 10.1016/S2468-1253(18)30118-3. [DOI] [PubMed] [Google Scholar]
- 93. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naive chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther 2018; 47: 259–267. [DOI] [PubMed] [Google Scholar]
- 94. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with sofosbuvir and velpatasvir, with or without voxilaprevir. Clin Gastroenterol Hepatol 2018; 16: 567–574 e6. [DOI] [PubMed] [Google Scholar]
- 95. Struble K, Chan-Tack K, Qi K, Naeger LK, et al. Benefit-risk assessment for sofosbuvir/velpatasvir/voxilaprevir based on patient population and hepatitis C virus genotype: US Food and Drug Administration’s evaluation. Hepatology 2018; 67: 482–491. [DOI] [PubMed] [Google Scholar]
- 96. Wyles D, Weiland O, Yao B, et al. Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C infection. J Hepatol 2018; 68: S23–S24. [DOI] [PubMed] [Google Scholar]
- 97. De Ledinghen V, Varaut A, Bedoya JU, et al. Sofosbuvir plus glecaprevir/pibrentasvir in patients with difficult to treat HCV infection. Final resuts of the French compassionate use. J Hepatol 2018; 68: S529. [Google Scholar]