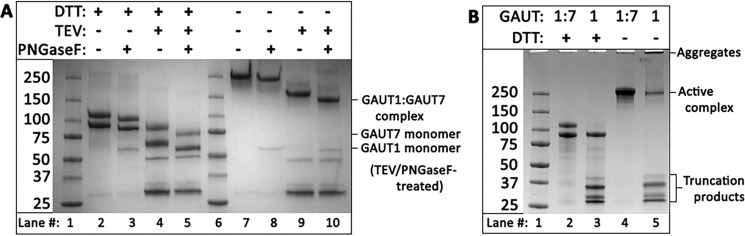
Figure 1.
Heterologous expression of the GAUT1:GAUT7 complex. A, Coomassie Blue–stained SDS-polyacrylamide gel of purified GAUT1Δ167 co-expressed with GAUT7Δ43 detected under reducing (+DTT, lanes 2–5) and nonreducing (−DTT, lanes 7–10) conditions. Incubation with TEV protease (+TEV, lanes 4 and 5 and lanes 9 and 10) removes N-terminal GFP/His8 tags, which electrophorese as ∼30 kDa bands post-cleavage. Incubation with PNGase F (+PNGase F, lanes 3, 5, 8, and 10) removes N-glycosylation. After 6 days of incubation, medium from transfected HEK293F cells was purified by nickel-affinity chromatography, protein concentration was quantified by UV-visible spectroscopy, and 4 μg of purified protein was separated by SDS-PAGE after overnight incubation with or without TEV and PNGase F. The locations of the GAUT1:GAUT7 complex after TEV and PNGase F treatment under nonreducing conditions and the GAUT1 and GAUT7 monomers under reducing conditions are designated on the right. B, Coomassie Blue–stained SDS-polyacrylamide gel of purified GAUT1 (lanes 3 and 5) compared with the GAUT1:GAUT7 complex (lanes 2 and 4). Proteins were detected under reducing (+DTT, lanes 2 and 3) and nonreducing (−DTT, lanes 4 and 5) conditions.