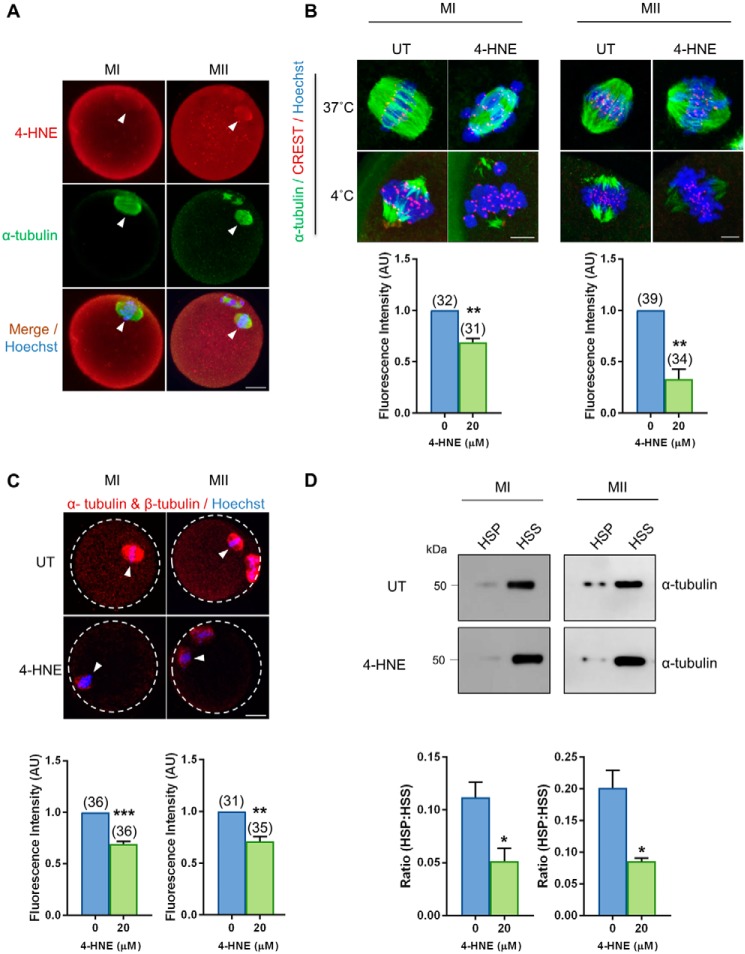
Figure 2.
Acute exposure to 4-HNE at prophase I arrest reduces kinetochore–microtubule stability and tubulin polymerization. GV oocytes were treated with 20 μm 4-HNE for 2 h, underwent IVM to MI or MII, and were prepared for immunofluorescence analysis, tubulin polymerization assays, and PLA alongside their untreated (UT) counterparts. A, 4-HNE was localized to the spindle of MI and MII oocytes using anti-4-HNE (red) and anti-tubulin (green) antibodies. Nuclei were counterstained with Hoechst (blue). Scale bar = 20 μm. B, MI and MII oocytes were also fixed following a cold shock at 4 °C for 7 min to examine k-mt stability. Immunofluorescence analysis using anti-α-tubulin (green), anti-CREST (red), and Hoechst (blue) revealed a significant decrease in the presence of stable k-mt in the spindles of 4-HNE–exposed oocytes. Scale bar = 5 μm. C, PLA with anti-α-tubulin and anti-β-tubulin antibodies (red) revealed a decrease in tubulin polymerization in MI and MII oocytes following acute 4-HNE exposure. Nuclei were counterstained with Hoechst (blue). Scale bar = 20 μm. immunofluorescence experiments were repeated across three independent biological replicates, with a minimum of 10 oocytes, pooled from a minimum of three animals. D, decrease in the ratio of polymerized (high speed pellet; HSP) versus free tubulin (high speed supernatant; HSS) was also detected in MI and MII oocytes after 4-HNE exposure, using a microtubule/tubulin in vivo assay. In vivo microtubule/tubulin assays were performed in biological and technical triplicate using 150 oocytes pooled from a minimum of four young animals. Samples were resolved on the same gel and transferred to the same membrane for immunoblot analysis and have been cropped for presentation. Statistical analyses were performed using Student's t test, *, p ≤ 0.05; **, p ≤ 0.01; and ***, p ≤ 0.001. Data are presented as mean of three replicates ± S.E. AU, arbitrary units.