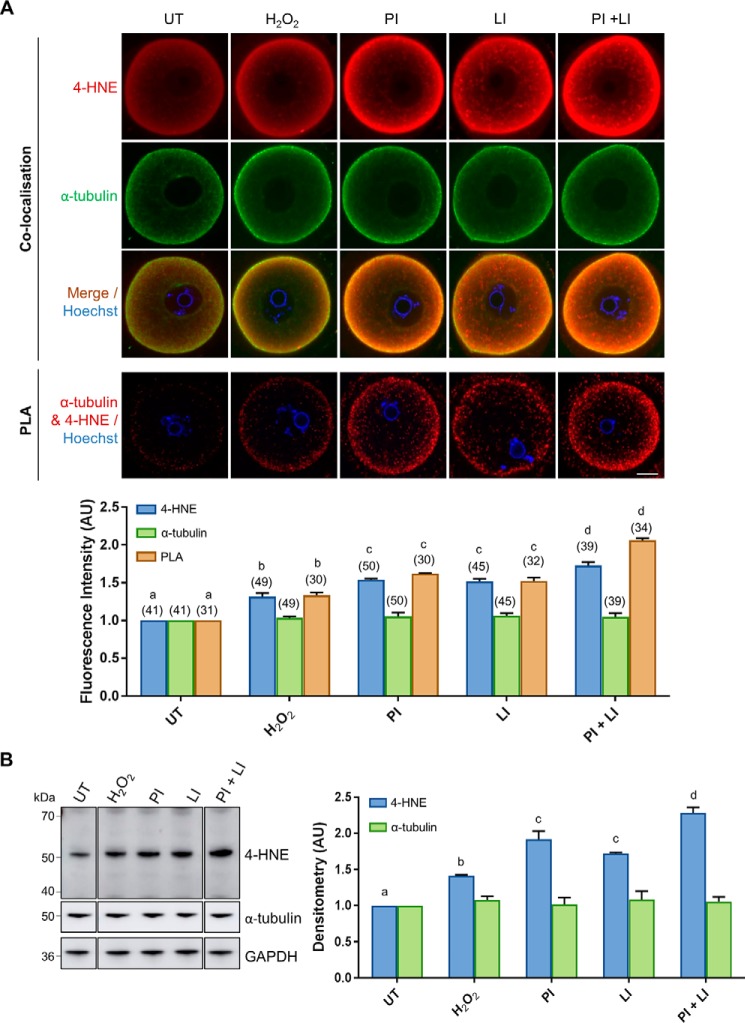
Figure 3.
Degradation of 4-HNE–modified α-tubulin is mediated by the proteasomal and lysosomal systems under conditions of oxidative stress. GV oocytes were treated with 35 μm H2O2 (H2O2) for 1 h to induce oxidative stress and then incubated in the presence or absence of the proteasome inhibitor MG132 (50 μm; PI) and/or the lysosomal inhibitor chloroquine (100 μm; LI) for 6 h. Oocytes were then prepared for immunofluorescence analysis, PLA, and immunoblotting analysis alongside their untreated (UT) counterparts to determine the degradation pathway for 4-HNE–modified α-tubulin. A, immunofluorescence analysis using anti-α-tubulin (green) and anti-4-HNE (red) antibodies revealed consistent levels of α-tubulin expression throughout all treatments groups, which co-localized the significantly increasing expression 4-HNE, particularly at the periphery of the oocyte under conditions of oxidative stress and proteasome and lysosome inhibition (ANOVA; p ≤ 0.0190). Similarly, PLA also indicated a significant increase in the 4-HNE modification of α-tubulin at the periphery of the oocyte under conditions of oxidative stress and proteasome and lysosome inhibition (ANOVA; p ≤ 0.0066). Nuclei were counterstained with Hoechst (blue). Scale bar = 20 μm. These experiments were repeated across three independent biological replicates, with a minimum of 10 oocytes, pooled from a minimum of three animals. B, consistent expression of α-tubulin with the increase in 4-HNE modification of α-tubulin upon proteasome and lysosome inhibition was also confirmed via immunoblotting with anti-4-HNE and anti-α-tubulin antibodies and was quantified for treated cell lysates against their untreated counterparts for reference (ANOVA; p ≤ 0.0377). Immunoblots were run on the same gel and transferred onto the same membrane for immunoblot analysis and have been cropped for presentation. Only the 50-kDa α-tubulin band was used for densitometry analysis. Immunoblots were stripped and re-probed with anti-GAPDH antibodies as a loading control. Immunoblots were performed in biological and technical triplicate using 100 oocytes per lane pooled from a minimum of three animals. Data are presented as mean of three replicates ± S.E. AU, arbitrary units.