Figure 3.
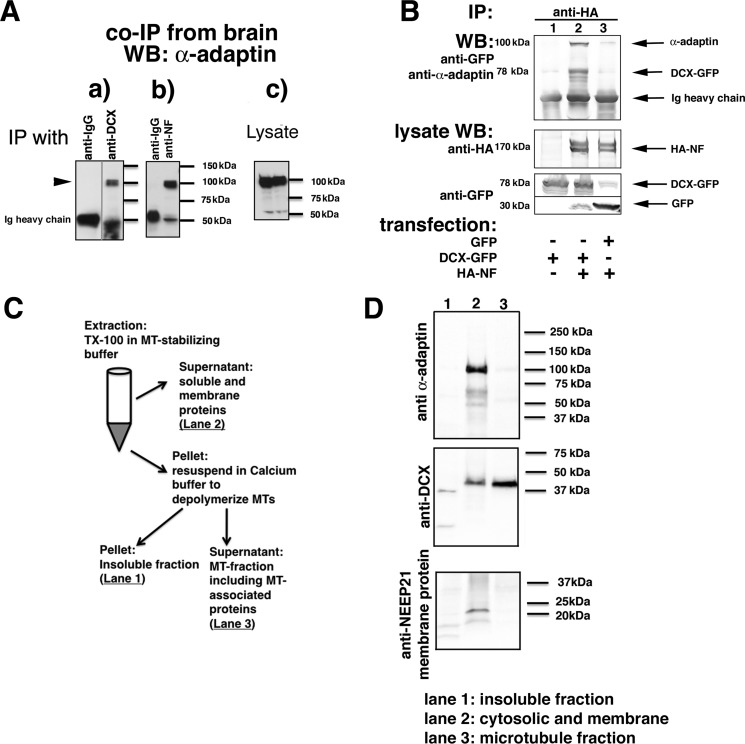
A complex of NF, DCX, and AP-2 can be immunoprecipitated from transfected cells and brain. A, α-adaptin is immunoprecipitated with anti-DCX antibodies from embryonic rat brain membrane fractions (a) as well as by anti-NF antibodies (b). Nonimmune IgG-coated beads were used as negative controls. Levels of endogenous α-adaptin are shown in the lysate blots (c). An intervening irrelevant lane was removed in a. B, α-adaptin is only co-immunoprecipitated with HA-NF if DCX-GFP is co-expressed. HEK293 cells were transfected with the indicated plasmids, and immunoprecipitations (IP) were carried out as labeled. Immunoprecipitates were analyzed by Western blotting (WB) with the indicated antibodies. The lysate (5% of total) was probed by Western blotting as labeled. C and D, freshly dissociated E18 rat cortex was homogenized and fractionated according to the diagram (shown in C) into a soluble (cytosolic and membrane proteins), MT-associated, and insoluble fraction. The insoluble (lane 1), soluble/membrane (lane 2), and microtubule-associated (lane 3) fractions were separated by SDS-PAGE and blotted against α-adaptin, DCX, and a membrane protein (NEEP21).