Figure 1.
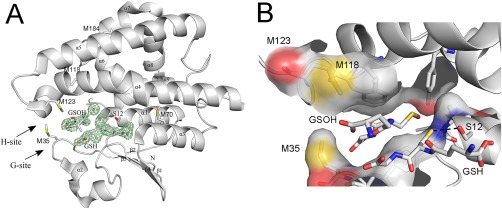
Crystal structure of reduced GSTF9. (A) The X‐ray crystal structure of reduced GSTF9red shows a typical GST‐fold. The crystal structure of GSTF9red is shown in a gray cartoon representation with the alpha helices and beta sheets labeled. The glutathione (GSH) binding site (G‐site) and the hydrophobic substrate‐binding site (H‐site) are indicated with arrows. A glutathione molecule is observed in the G‐site, and sulfenylated GSH (GSOH) in the H‐site. The catalytic serine (S12), the Met residues (M35, M70, M118, M123 and M184), and the N‐ and C‐terminus are indicated. An omit map contouring the GSH and GSOH at 3σ is shown in green. (B) A closer view of the three methionines (M35, M118 and M123) located close to the G‐ and H‐site. Surface representation is shown for atoms within 4 Å of H‐GSOH. The H‐bond between the catalytic S12 and the sulfur of GSH is 3.3 Å (black dotted line).
