Figure 2.
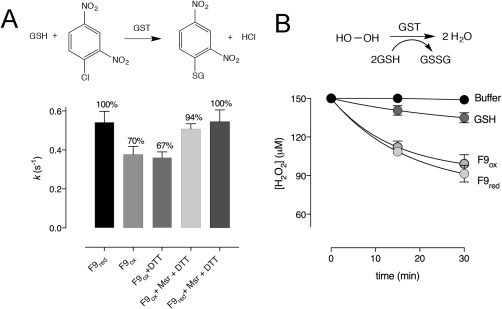
GSTF9 oxidation has a different impact on the transferase and glutathione peroxidase activity. (A) Bar graphs show the percentage of transferase activity measured in rate constants (k) of GSTF9red and GSTF9ox (0.2 µM), where the GST catalysed conjugation of GSH (0.5 mM) onto CDNB (3.8 mM) is followed at 340 nm. When oxidized, GSTF9ox activity significantly (P < 0.001) decreases from 100% (0.54 s−1) to 70% (0.38 s−1). MsrA and MsrB treatment causes activity increase from 70% to 94% (0.51 s−1), indicating that Msr enzymes are capable of recovering GSTF9ox activity. Two control samples were used: GSTF9ox incubated with DTT and GSTF9red incubated with MsrA, MsrB and DTT. The data from at least 3 independent experiments are presented as a mean ± SD. (B) FOX assay was used to compare the peroxidase activity of GSTF9red and GSTF9ox in the presence of 150 µM H2O2. Remained H2O2 concentrations were determined for samples taken after 15 and 30 min. Upon treatment of GSTF9 with hydrogen peroxide (GSTF9ox), glutathione peroxidase activity is not influenced. As a control, the influence of GSH on H2O2 reduction was also recorded. The data from 3 independent experiments were normalized and presented as a mean ± SD.
