Figure 11.
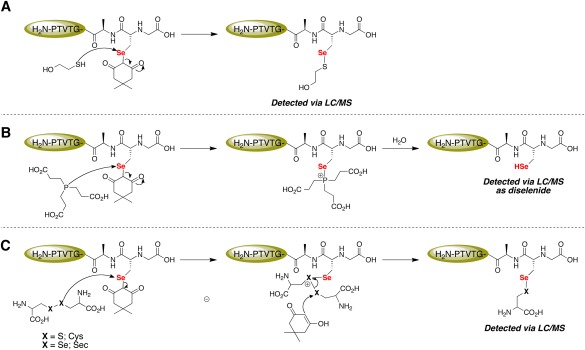
Mechanistic explanation for the lability of the Sec‐dimedone label. (A) The thiol moiety of βME attacks the electrophilic Se atom of Sec and dimedone acts as the leaving group. (B) TCEP acts as the nucleophile and attacks the electrophilic Se atom of Sec and dimedone acts as the leaving group. The nascent phosphonium ion is then rapidly hydrolyzed to yield a phosphine oxide and free Sec‐selenol residue. (C) The selenium atom of Sec again acts as the electrophile and is attacked by the disulfide/diselenide moiety of either cystine or selenocystine, releasing dimedone. Dimedone, as the enol, then attacks the electrophilic sulfur/selenium atom of the cationic intermediate, resulting in the formation of a mixed selenosulfide/diselenide.
